Infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can result in asymptomatic to severe coronavirus disease 2019 (COVID-19). There is high variability in the manifestation of the disease and the generation of an immune response by the host.
 Study: The β-NGF/TrkA signalling pathway is associated with the production of anti- nucleoprotein IgG in convalescent COVID-19. Image Credit: Adao / Shutterstock.com
Study: The β-NGF/TrkA signalling pathway is associated with the production of anti- nucleoprotein IgG in convalescent COVID-19. Image Credit: Adao / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Immune response and COVID-19 severity
The wide range of severity of COVID-19 has been correlated with the humoral response, which is often attributed to the levels and duration of specific antibodies circulating in the serum. Some studies have shown that differences in the clinical manifestations of COVID-19 are due to age, ethnicity, and pre-existing medical conditions. The molecular mechanisms leading to the humoral responses are currently being researched; however, these studies focus on individuals with severe COVID-19.
Although most SARS-CoV-2 infected individuals mount a specific humoral response, between 1% and 10% of COVID-19 patients have very low anti-spike (S) or anti-nucleoprotein (N) immunoglobulin G (IgG) antibodies.
Similarly, a majority of COVID-19 convalescents have SARS-CoV-2-specific T-cells in their serum. This is the case even in individuals with undetectable humoral responses.
One study has shown that an early T-cell response during SARS-CoV-2 infection is associated with milder symptoms and rapid viral clearance. Likewise, early response dominated by anti-N antibodies is associated with severe COVID-19, whereas an early immune response that is accompanied by a greater amount of anti-S antibodies is associated with mild to moderate manifestations.
A cross-sectional study
In the current study, the researchers randomly selected 40 donors and created four sex- and age-matched groups according to polymerase chain reaction (PCR) test results and anti-N antibody status.
Group 1 had 10 unexposed healthy individuals with negative PCR and negative anti-N IgG, whereas Group 2 consisted of 10 individuals with positive PCR and positive anti-NP IgG. Group 3 had 7 individuals with positive PCR and negative anti-N IgG, whereas Group 4 had 13 individuals with negative or unavailable PCR results and positive anti-N IgG. These individuals were sampled immediately following the first wave of the COVID-19 pandemic in the U.K. Of the seven PCR positive and anti-N IgG negative individuals, only four gave consent for additional peripheral mononuclear cells (PBMC) isolation.
The investigators analyzed serum samples and PBMC. Serum analysis would give an estimation of the antibody levels and cytokine levels related to infection and inflammation.
PBMC analysis would assess the presence and activity of antigen-specific T-cells. The virus-specific T-cell response was determined by interferon γ (IFN-γ) enzyme-linked immune absorbent spot (ELISPOT) and flow cytometry assays after incubation of PBMCs with pools of selected SARS-CoV-2 specific peptides.
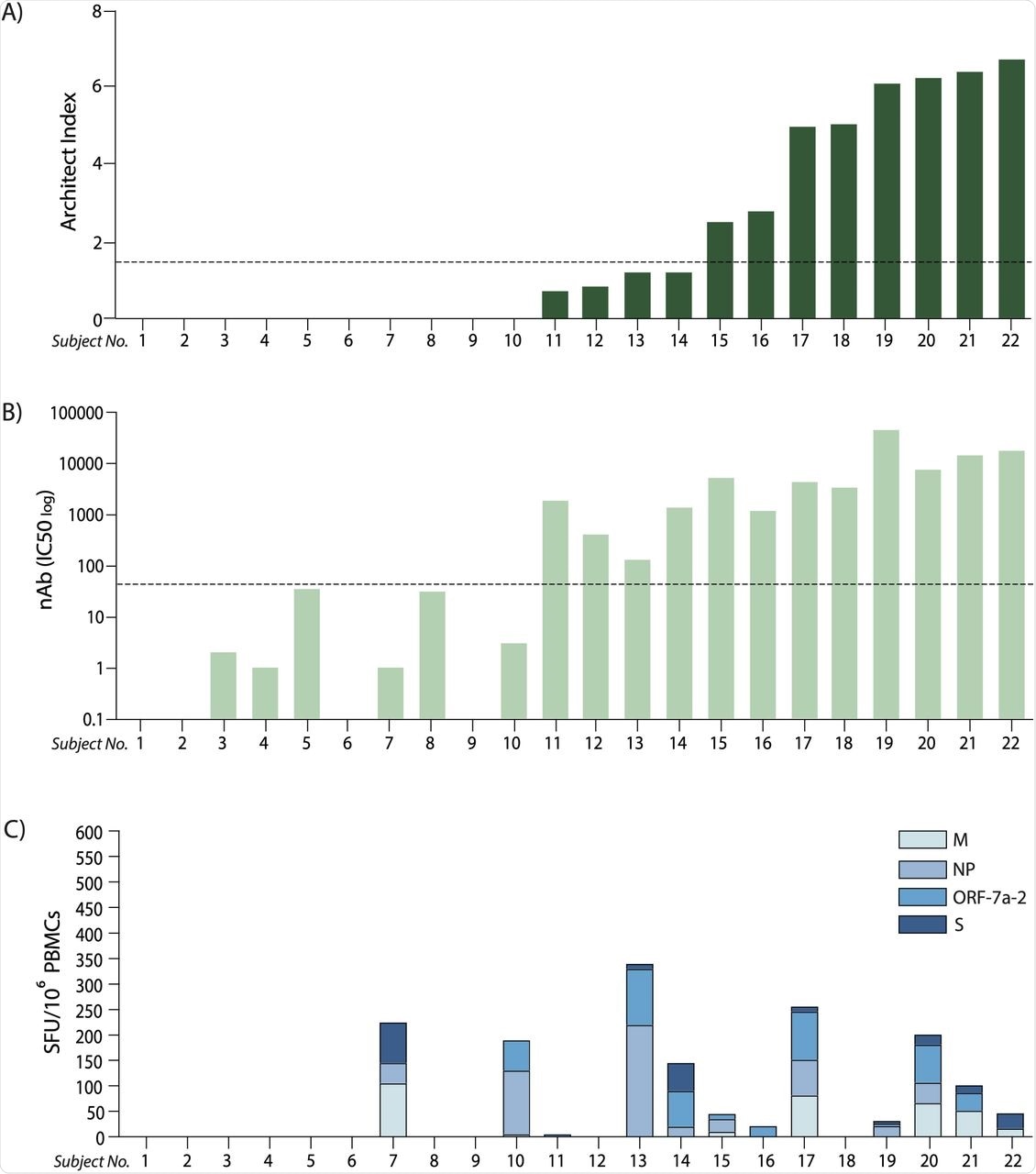 (A) Anti-nucleoprotein IgG levels expressed as Architect Index (manufacturer arbitrary units) for each subject analysed (ordered lowest to highest level); the dotted line represents the 1.4 cut-off, below which samples are considered as negatives. (B) Neutralising antibody (nAb) titres (IC50) corresponding to the same subjects in (A); the dotted line represents the cut-off below which samples are considered as negatives (IC50<50). (C)Cumulative T cell response to the four peptide pools derived from SARS-CoV-2 expressed as spot-forming units (SFU) of IFN-γ-secreting cells after 16-hour stimulation, ordered corresponding to the subjects in (A) and (B). (M: membrane; NP: nucleoprotein; ORF-7a-2: open reading frame 7a-2; S: spike).
(A) Anti-nucleoprotein IgG levels expressed as Architect Index (manufacturer arbitrary units) for each subject analysed (ordered lowest to highest level); the dotted line represents the 1.4 cut-off, below which samples are considered as negatives. (B) Neutralising antibody (nAb) titres (IC50) corresponding to the same subjects in (A); the dotted line represents the cut-off below which samples are considered as negatives (IC50<50). (C)Cumulative T cell response to the four peptide pools derived from SARS-CoV-2 expressed as spot-forming units (SFU) of IFN-γ-secreting cells after 16-hour stimulation, ordered corresponding to the subjects in (A) and (B). (M: membrane; NP: nucleoprotein; ORF-7a-2: open reading frame 7a-2; S: spike).
Antibody levels and T-cell responses
Seven out of the 17 convalescents had undetectable levels of anti-N IgG, irrespective of the symptoms presented.
The individuals that did not produce anti-N antibodies were not able to produce IFN-γ after stimulation with peptides derived from the S protein. However, two out of the four anti-N individuals produced a detectable IFN-γ response after stimulation with peptides derived from N.
Anti-N antibody levels are not indicative of effective immunity. Therefore, the investigators analyzed the levels of neutralizing antibodies.
A positive correlation was observed between anti-N antibody levels and the levels of neutralizing antibodies. However, five out of seven anti-N negative individuals showed evidence of neutralizing activity. Similarly, a discrepancy was noted between antibody level and T-cell response.
The investigators further characterized the T-cell response by analyzing the production of four effector cytokines. The frequencies of antigen-specific T-cells were similar between the groups. However, the T cells from anti-N positive and negative subjects reacted differently to the peptide pools in terms of the number of cytokines produced after stimulation. This suggests that a differential hierarchy of cytokine response exists among convalescent individuals.
The investigators then analyzed a panel of cytokines related to infection and inflammation from the serum of individuals to determine if these factors govern anti-N production. The anti-N positive and anti-N negative individuals differed in the levels of only two serum cytokines including β nerve growth factor (β-NGF) and interleukin- 1α (IL-1α). Furthermore, β-NGF significantly correlated with anti-NP positivity. The T-cells of anti-N negative subjects expressed lower amounts of the β-NGF-specific receptor tropomyosin receptor kinase A (TrkA).
Limitations of the study
PCR testing was not widely available during the early phase of the pandemic. Therefore, the time frame between symptom onset, PCR test, and sample collection could not be determined for all individuals participating in the study. The sample size was limited.
Conclusion
The current study identifies an association between the presence of anti-N antibodies and the β-NGF/TrkA pathway, which is active in lymphocytes and is involved in inflammatory conditions of the airways. The investigation of the mechanistic regulation of this pathway in COVID-19 is required to determine an influence on the durability of the T-cell response and vaccine-induced immunity.
Characterization of these mechanisms will help to assess the relative risk of developing severe disease. This, in turn, will inform to prioritize future treatment and vaccination strategies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Usai, C., Gibbons, J. M., Pade, C., et al. (2021) The β-NGF/TrkA signalling pathway is associated with the production of anti- nucleoprotein IgG in convalescent COVID-19. medRxiv. doi:10.1101/2021.11.11.21266223. https://www.medrxiv.org/content/10.1101/2021.11.11.21266223v1
- Peer reviewed and published scientific report.
Usai, Carla, Joseph M. Gibbons, Corinna Pade, Wenhao Li, Sabina R. M. Jacobs, Áine McKnight, Patrick T. F. Kennedy, and Upkar S. Gill. 2022. “The β-NGF/TrkA Signalling Pathway Is Associated with the Production of Anti-Nucleoprotein IgG in Convalescent COVID-19.” Frontiers in Immunology 12 (January). https://doi.org/10.3389/fimmu.2021.813300. https://www.frontiersin.org/articles/10.3389/fimmu.2021.813300.