Globally, the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has destabilized healthcare systems and economies. Recent advances in understanding the structural biology and pathology of SARS-CoV-2 have led to the development and emergency approval of several COVID-19 vaccines, as well as a variety of candidate vaccines that are still in development.
Background
Amongst recently developed SARS-CoV-2 candidate vaccines, natural protein-based nanoparticles that are well adapted for multivalent antigen presentation and improved immune activation are being explored for their ability to trigger powerful humoral and cellular immune responses.
In fact, a Phase III clinical trial established the safety and efficacy of the Novavax, which is a recombinant nanoparticle vaccine composed of a stabilized version of the SARS-CoV-2 spike (S) protein. Furthermore, Phase I clinical trials with ferritin-based protein nanoparticles, containing the SARS-CoV-2 S protein are underway (NCT04784767).
By displaying multivalent antigens on the surface of soluble proteins, these nanoparticle-based vaccine technologies can increase the immunogenicity and stability of soluble antigens. They can trigger a variety of immunological activities including the efficient transport of antigens to the lymph nodes, retention of follicular dendritic and helper T-cells, as well as the formation, activation, and expansion of B-cells, including memory B-cells and long-lived plasma cells.
Protein-based nanoparticles have minimal biosafety or environmental concerns related to their manufacturing process and could prove to be more accessible to the general public due to lower production costs. Thus, protein-based nanoparticles have been extensively exploited as a vehicle for the delivery of a variety of vaccines and pharmaceuticals.
A recent review published in the International Journal of Molecular Sciences discusses recent advances in protein-based nanoparticle vaccines against SARS-CoV-2.
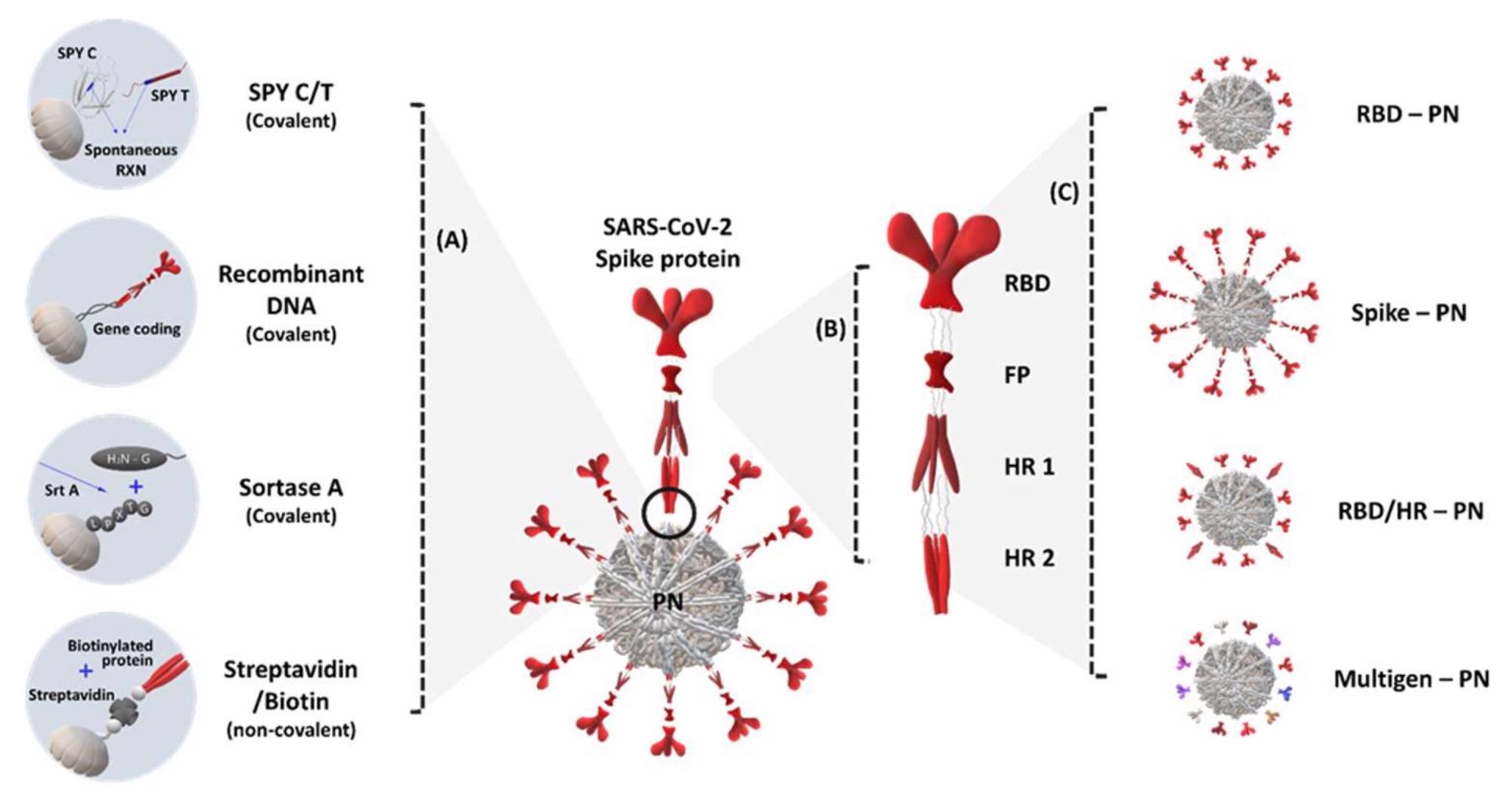
Application of protein nanoparticles (PN) that elicit severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)- and SARS variant-specific immune response: (A) methods for ligating PN components, nanoparticles, and immunogens based on covalent (SpyCatcher/SpyTag, recombinant DNA, and sortase-mediated bonding) and non-covalent (streptavidin/biotin) interactions; (B) schematic structure of the SARS-CoV-2 spike (S) protein; (C) type of immunogen applications for displaying PNs from the partial epitope region of SARS-CoV-2 to other types of receptor-binding domains (RBDs) and S proteins; SPY C/T, SpyCatcher/SpyTag; HR, heptad repeat.
About the study
The current review illustrates the design and method for displaying surface antigenic domains of various protein-based nanoparticles, as well as the performance of the produced nanoparticle-based vaccinations. The primary focus was on major developments in protein-based nanoparticle vaccines for SARS-CoV-2 protection. The current review also discusses the design and techniques used for incorporating antigenic domains, such as the spike (S) protein, the receptor-binding domain (RBD), and other domains into various protein nanoparticles.
The effectiveness of these synthetic protein nanoparticle vaccines against SARS-CoV-2 infection in mice, human angiotensin-converting enzyme 2 (ACE2) transgenic mice, rabbits, hamsters, ferrets, and macaques was highlighted, in addition to their ability to protect against additional Coronaviridae viruses.
Study findings
The findings of the current study provided an overview and context for recent breakthroughs in protein nanoparticle vaccines that have been subjected to clinical trials. The review also evaluates recent clinical trial developments and presented an outlook for protein-based nanoparticle vaccines.
It was noted that both Astra Zeneca and Johnson & Johnson have delivered viral vector vaccines internationally, while Pfizer and Moderna have distributed messenger ribonucleic acid (mRNA)-based vaccines, and Sinovac Biotech has distributed inactivated vaccines.
Hundreds of SARS-CoV-2 vaccine candidates are currently undergoing clinical studies. Among these, recombinant protein subunit vaccines based on the SARS-CoV-2 S protein or RBD are an appealing option to inactivated, viral vector, or mRNA-based vaccinations due to their proven safety. Despite intensive efforts to create and utilize S proteins or RBD-based subunits as vaccine candidates, their poor immunogenicity continues to be a barrier.
In comparison to monomeric S protein subunit vaccines, nanoparticle-based vaccinations are capable of displaying either multivalent S protein or RBD. This repeating pattern induces a variety of immunological processes, including strong B-cell activation, memory B-cell growth, and follicular dendritic cell retention. Thus, protein nanoparticle-based vaccines are associated with a potential for greater effectiveness, neutralizing antibody responses, and specific humoral and cellular immune responses as compared to S protein subunit vaccines at lower dosages.
Additionally, the intrinsic stability of protein nanoparticle-based SARS-CoV-2 vaccines, the absence of a rigorous cold-chain supply chain, and the absence of any unique biosafety environment concerns in manufacturing operations with lower production costs are significant advantages.
On the other hand, vaccine platforms based on protein nanoparticles induce high cross-reactive immunity against developing SARS-CoV-2 variants of concern (VOC) and other zoonotic coronaviruses such as SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV).
Study takeaways
Despite the fact that mRNA-based vaccines from Pfizer and Moderna, as well as viral vector vaccines from Astra Zeneca and Johnson & Johnson, have demonstrated significant protective efficacy against the original Wuhan-Hu-1 strain of SARS-CoV-2with slowed infection rates, the growing prevalence of rapidly evolving SARS-CoV-2 VOCs, such as Delta and Mu variants, poses new challenges. Moreover, two additional zoonotic beta-coronaviruses, SARS-CoV and MERS-CoV, have emerged in human populations over the last two decades, and the likelihood of future zoonotic coronavirus emergence persists, thus forecasting the next pandemic.
In this regard, protein nanoparticle-based vaccines enable the development of a next-generation vaccine platform that is capable of defending against SARS-CoV-2 VOCs and other zoonotic coronaviruses. These may offer significant protection against the devastation caused by global pandemics to public healthcare systems and economies.
Journal reference:
- Sung, H. D., Kim, N., Lee, Y., & Lee, E. J. (2021). Protein-Based Nanoparticle Vaccines for SARS-CoV-2. International Journal of Molecular Sciences. doi:10.3390/ijms222413445.