The United States Food and Drug Administration (FDA) has authorized three vaccines against coronavirus disease 2019 (COVID-19), all of which have been found to be safe and highly efficacious in preventing symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Breakthrough infections, which are defined as infections that occur in vaccinated individuals, are common with both COVID-19 vaccines and many other vaccines. However, excessive breakthrough infections may suggest a possible lack of vaccine effectiveness.
It is essential to determine the efficacy or the lack of effectiveness of currently available COVID-19 vaccines in order to prevent the occurrence of breakthrough infections. Person-generated real-world data or patient-reported outcomes can help to better understand and characterize breakthrough infections.
About the study
In a new study published on the medRxiv* preprint server, researchers describe the pattern and characteristics of infections after COVID-19 vaccination in individuals who received any of the three COVID-19 vaccines currently authorized for use in the United States. These include the Pfizer-BioNTech BNT162b2, Moderna mRNA-1273, and Johnson & Johnson Ad26.COV2.S vaccines. The researchers also included individuals who were never vaccinated in this study.
All data were extracted from the COVID-19 Active Registry Experience (CARE). CARE is a web-based registry that was launched to study COVID-19 symptoms and severity outside of the hospital setting, to understand the factors that could mitigate the impact of the SARS-CoV-2 infection.
The selected cohort consisted of 10,412 participants, including 8,554 who had ever received a U.S. FDA-authorized vaccine, as well as 1,858 who were never vaccinated but had had a positive COVID-19 test. Among the vaccinated, 7,532 had completed the vaccination doses.
Study findings
The average age of the vaccinated participants was 47.9 years, of which more than 83% of the participants were female. Among those who had ever received an authorized vaccine, 1,160 reported a prior SARS-CoV-2 infection before being vaccinated against COVID-19. Meanwhile, among the fully vaccinated, 74 reported breakthrough infections.
Overall, 2.3% of participants acquired SARS-CoV-2 infection during the interim period, which is defined as the time between two vaccine doses of either the BNT162b2 or mRNA-1273 vaccines. Among those who reported breakthrough infections after full vaccination, the median time to a positive COVID-19 test was 104.5 days, which was comparable across all vaccine manufacturers.
The average age of the people who were infected after complete vaccination was 44.5 years. Among the individuals who got infected after partial vaccination, the median time from the first dose to infection was eight days.
It was noted that individuals who were fully vaccinated reported lower proportions of each COVID-19 symptom as compared to the partially vaccinated and unvaccinated groups. Some of the symptoms that were commonly reported included headache, aches and pains, decreased sense of taste and smell, chills, and diarrhea.
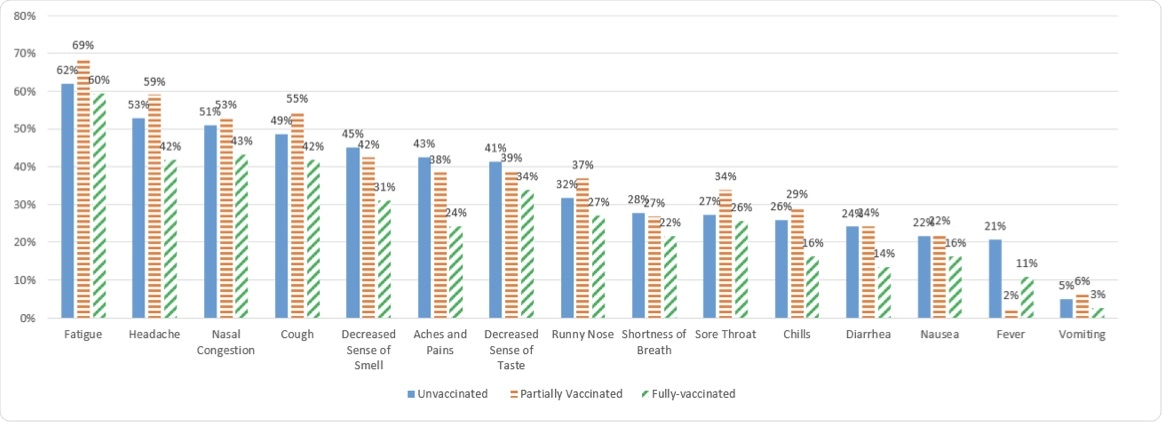
Symptoms reported by vaccination status.
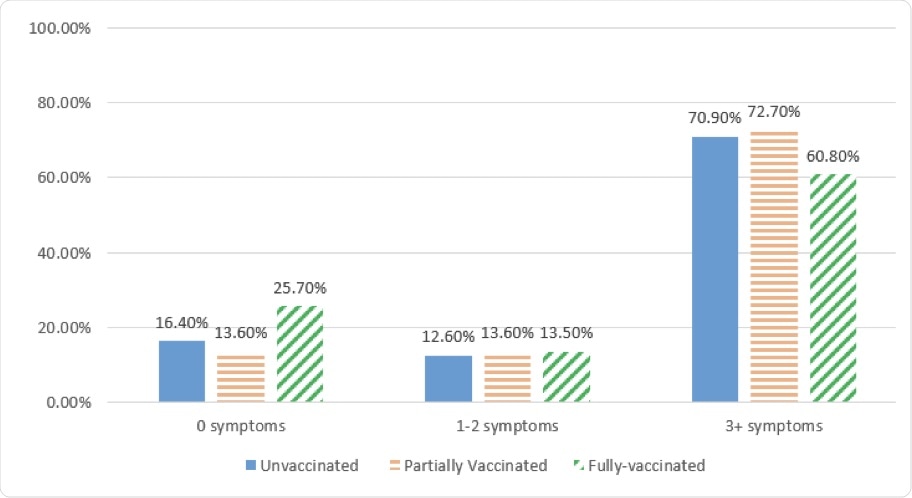
Number of symptoms reported by vaccination status.
Notably, 25.7% of fully vaccinated breakthrough cases reported no symptoms. The rates of asymptomatic breakthrough infections were 13.6% and 16.4% among partially vaccinated and unvaccinated cases, respectively.
Moreover, 97.3% of fully vaccinated individuals who had breakthrough infections reported only mild symptoms. The rate of occurrence of moderate or severe symptoms was higher among those who were partially vaccinated or unvaccinated.
Conclusions
Taken together, the current study findings reveal that only a small proportion of vaccinated individuals acquire breakthrough infections after complete vaccination. The majority of these breakthrough infections are asymptomatic, with some presenting with fewer and milder symptoms as compared to when unvaccinated people present with COVID-19.
It should be noted that although this study found that the occurrence of breakthrough infections is rare, the recent dominance of the SARS-CoV-2 Omicron variant may have altered the rarity of this event.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Reynolds, M., Xie, Y., Knuth, K., et al. (2022). COVID-19 vaccination breakthrough infections in a real-world setting: Using community reporters to evaluate vaccine effectiveness. medRxiv. doi:10.1101/2022.01.11.22268736d. https://www.medrxiv.org/content/10.1101/2022.01.11.22268736v1
- Peer reviewed and published scientific report.
Reynolds, Matthew W, Yiqiong Xie, Kendall B Knuth, Christina D Mack, Emma Brinkley, Stephen Toovey, and Nancy A Dreyer. 2022. “COVID-19 Vaccination Breakthrough Infections in a Real-World Setting: Using Community Reporters to Evaluate Vaccine Effectiveness.” Infection and Drug Resistance Volume 15 (September): 5167–82. https://doi.org/10.2147/idr.s373183. https://www.dovepress.com/covid-19-vaccination-breakthrough-infections-in-a-real-world-setting-u-peer-reviewed-fulltext-article-IDR.