
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Older age is a common risk factor for severe disease and mortality following SARS-CoV-2 infection. Thus, elderly individuals have been prioritized for both primary and booster vaccination against the coronavirus disease 2019 (COVID-19).
The emergence of the SARS-CoV-2 Omicron variant is of particular concern for older individuals as compared to younger individuals because there has been a greater amount of time since the elderly received their COVID-19 vaccine. While booster vaccines can induce Omicron-neutralizing activity, their long-term effects on the elderly are not yet clear.
About the study
The researchers of the present study longitudinally determined SARS-CoV-2 neutralizing activity of sera against different SARS-Cov-2 variants in a prospective cohort of 37 individuals with a median age of 82 years. All study participants were monitored for ten months after a two-dose BNT162b2 vaccination and for up to 4.5 months after receiving a single BNT162b2 booster vaccine dose.
Using a pseudovirus assay, the researchers determined 50% geometric mean inhibitory serum dilutions (GeoMean ID50) against the SARS-CoV-2 Wu01 vaccine strain, as well as the Delta and Omicron variants. Upon completion of the two-dose vaccine regimen, the researchers collected sera from the participants at one and five months.
Study findings
The results show that detectable neutralizing activity against Omicron was almost absent after two vaccine doses; however, these levels returned in 89% of the participants after booster immunization. Two BNT162b2 vaccine doses induced detectable neutralizing activity against the Wu01 and Delta strains at 95% and 84%, respectively, in most individuals, while neutralizing activity against Omicron was undetectable or only minimally detectable.
Over the four months of follow-up, neutralizing serum titers declined by six- and seven-fold, respectively, against Wu01 and Delta strains. The researchers obtained early post-boost sera samples one month after booster vaccination and found that booster immunization led to an over 50-fold increase in neutralizing activity against the Wu01 and Delta strains. Furthermore, booster immunization was found to induce a strong Omicron neutralizing activity in 89% of the elderly participants.
Follow-up samples obtained three to five months after the booster vaccination showed that neutralizing titers against the Wu01, Delta, and Omicron strains declined by 2.7-, 2.3-, and 3.0-fold, respectively. The team used linear mixed-effects models to determine the decline rate in neutralizing activity and found that neutralizing titers against the SARS-CoV-2 Wu01, Delta, and Omicron variants showed similar declines following booster dose. Furthermore, detectable activity was maintained against Omicron in 81% of individuals.
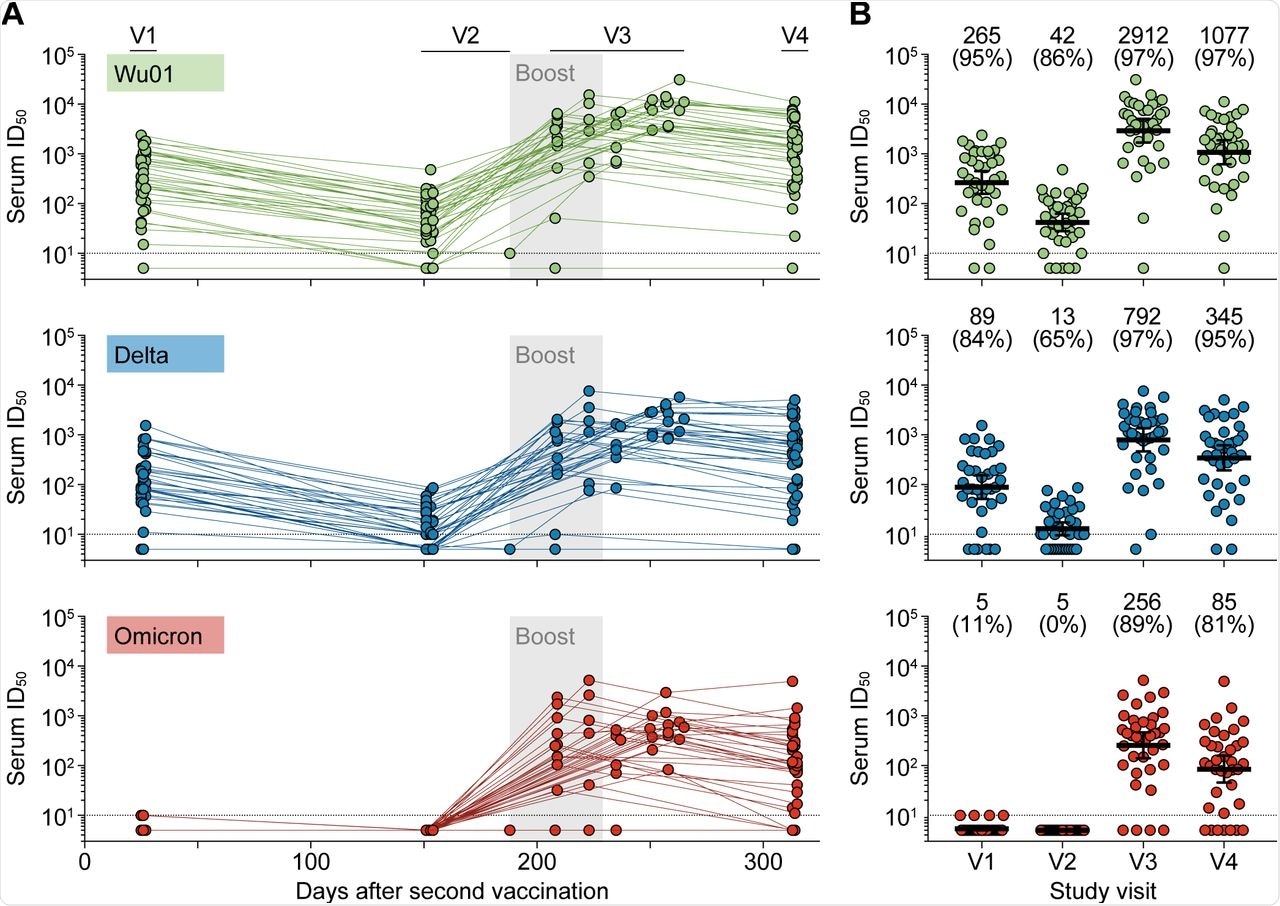
Longitudinal assessment of SARS-CoV-2-neutralizing serum activity in elderly individuals.
Conclusions
The current study demonstrates the effectiveness of mRNA booster doses in inducing neutralizing activity against the SARS-CoV-2 Omicron variant in the highly vulnerable elderly population. Furthermore, these findings provide crucial information on the durability of vaccine response.
Furthermore, booster immunizations were found to be highly valuable tools that can restore waning vaccine effectiveness and reduce the risk of severe disease in the elderly in the absence of vaccines specific to the Omicron variant. Taken together, these findings also show that previous knowledge about waning humoral immunity can guide vaccination strategies against the Omicron variant in the highly vulnerable elderly population.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Vanshylla, K., Tober-Lau, P., Gruell, H., et al. (2022). Durability of Omicron-neutralizing serum activity following mRNA booster immunization in elderly individuals. medRxiv. doi:10.1101/2022.02.02.22270302. https://www.medrxiv.org/content/10.1101/2022.02.02.22270302v1.full-text.
- Peer reviewed and published scientific report.
Vanshylla, Kanika, Pinkus Tober-Lau, Henning Gruell, Friederike Münn, Ralf Eggeling, Nico Pfeifer, N Han Le, et al. 2022. “Durability of Omicron-Neutralising Serum Activity after MRNA Booster Immunisation in Older Adults.” The Lancet Infectious Diseases 22 (4): 445–46. https://doi.org/10.1016/s1473-3099(22)00135-9. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00135-9/fulltext.