In a recent study posted to the medRxiv* preprint server, a team of researchers from the United States estimated the benefits of scaling up coronavirus disease 2019 (COVID-19) vaccination in the low- and lower-middle-income countries (LIC/LMICs) amid the global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant using economic and epidemiological models linearized with public data of COVID-19 vaccinations and deaths.

Study: Model-based estimates of deaths averted and cost per life saved by scaling-up mRNA COVID-19 vaccination in low and lower-middle income countries in the COVID-19 Omicron variant era. Image Credit: cortex-film/ Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Although reports show that over 60% of the world population has received a single dose of the COVID-19 vaccine, the global distribution of the COVID-19 vaccine is inequitable.
In low-income countries, only 4% of the population has received a full primary vaccination series as compared to 70% population in high-income countries.
These inequities will result in the worldwide delay in recovery from the ongoing pandemic and further serve as a ground to fuel the unstoppable emergence of SARS-CoV-2 variants.
In the current study, the researchers estimated the number of deaths that could be averted by scaling up COVID-19 vaccinations in LIC/LMICs.
Study Design
The researchers collected data of region-wise populations, vaccinations numbers, COVID-19 deaths, and excess deaths from 2020 and 2021 from data sources such as Our World in Data, World Health Organization (WHO), Institute for Health Metrics, and Evaluation, and The Economist, respectively.
Data regarding infection fatality rate (IFR) for the Omicron variant, immunity protection against mortality post-COVID-19 infection, vaccine effectiveness, and vaccine cost per dose were extracted from published literature. Only LICs and LMICs were included in the analysis based on the World Bank Designations. In addition, the researchers performed sensitivity analysis over a range of parameter estimates to account for uncertainty.
The researchers developed an analytical model to assess deaths averted, total COVID-19 vaccinations cost, cost averted per death, assuming 100% vaccination coverage in LICs/MICs. To estimate the cost and effect of global vaccinations, the team evaluated two potential dosage scenarios - two-dose scenarios (two doses of mRNA vaccines) and three-dose scenarios (full primary course with an additional booster).
Findings
The researchers observed that scaling up of vaccination program aiming to provide two and three doses of an mRNA vaccine to all individuals in LIC and LMIC would cost $35.5 billion and $61.2 billion, respectively, and would deter 1.3 and 1.5 million deaths due to COVID-19 with a cost aversion of $26,900 and $40,800 per death, respectively.
In the scenario of two-dose mRNA vaccination, at IFR 5/1,000, the cost per death averted was $4,500, and 95% vaccines effectiveness against mortality was achieved, while at IFR of 5/10,000, the cost per death averted and vaccine effectiveness against mortality was $53,800 and 80%, respectively. Likewise, at the same parameter estimates, the number of deaths averted ranged from 7.8 million to 0.7 million, respectively.
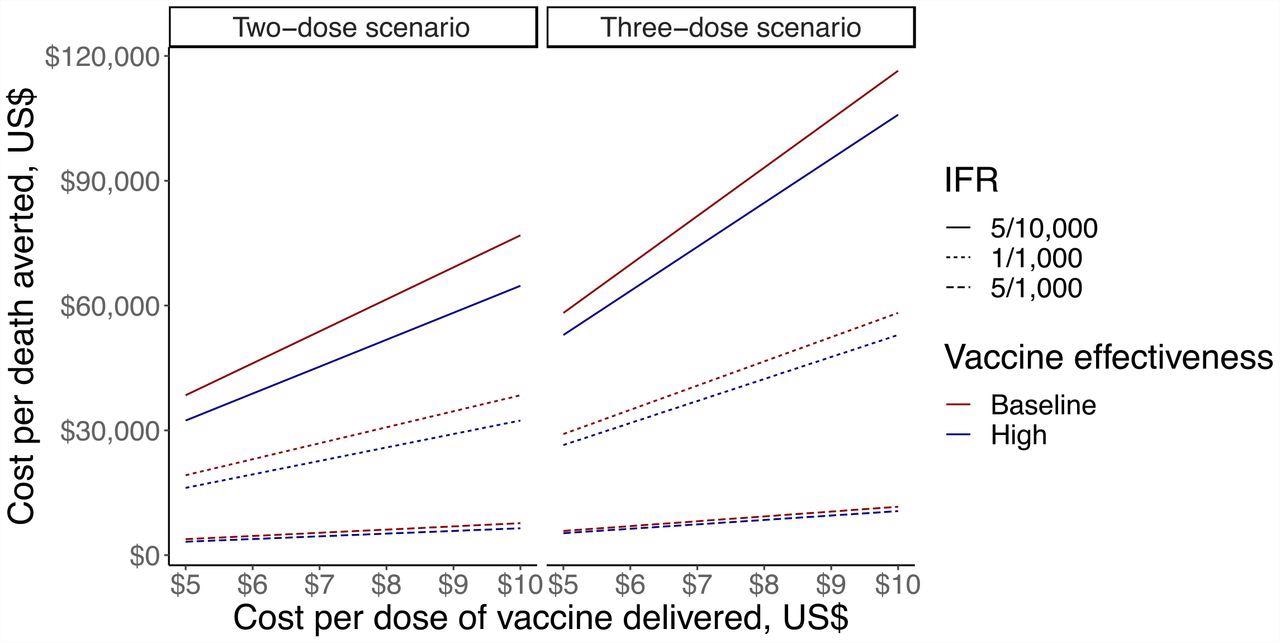
Sensitivity analysis looking at cost-per-death averted of vaccination in LIC/LMIC, ranging cost per vaccine dose, IFR and vaccine effectiveness against mortality in the two-dose scenario (first panel) and three-dose scenario (second panel). The y-axis shows cost-per-death averted in US$, the x-axis shows cost per dose of vaccine in US$. Solid lines show IFR of 5/10,000, dotted line shows IFR of 1/10,000, and dashed lines show IFR of 5/1,000. Dark red lines show baseline vaccine effectiveness (80% in two-dose scenario and 90% in three-dose scenario), and dark blue lines show high vaccine effectiveness (95% in two-dose scenario and 99% in three-dose scenario).
On the same note, the team analyzed that with the scenario of three-dose mRNA vaccination, at an IFR of 5/1,000, the cost per death averted was $74,000 and 99% vaccines effectiveness against mortality, while at IFR of 5/10,000 the cost per death averted was $81,500 and vaccine effectiveness against mortality was 90%. Likewise, at the same parameter estimates, the number of deaths averted ranged from 8.3 million to 0.8 million, respectively.
The team noted that in a two-dose mRNA vaccine scenario, on varying the cost of vaccine from $5 to $10 per dose, at an IFR of 1/1000 with 80% vaccine effectiveness, the cost-range per-death estimate was $19,200 to $38,400. In the three-dose scenario at the same parameters, the observed cost-range per-death estimate was $29,100 to $ 58,200 at a cost per dose of $5 and $10, respectively
The researchers noted that for the two-dose and three-dose scenarios, reducing 75% vaccine uptake while keeping 100% vaccine cost, an aversion of 1 million and 1.2 million deaths at $25,900 and $53,400 cost per death was observed, respectively.
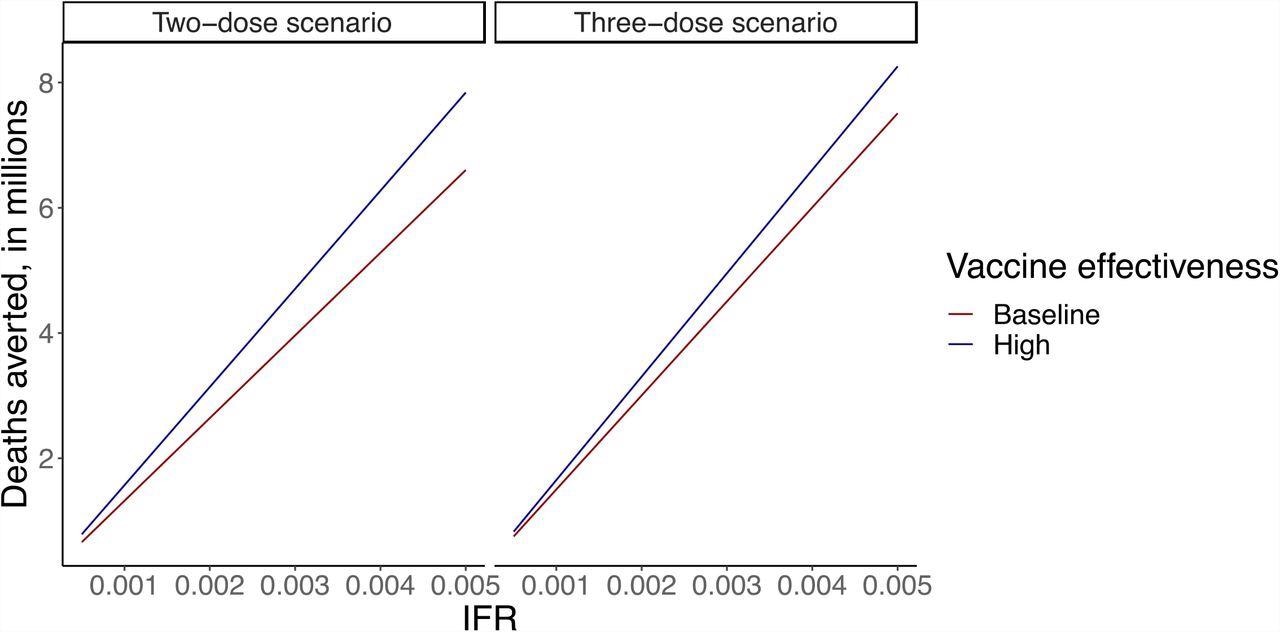
Sensitivity analysis looking at deaths averted of vaccination LIC/LMIC, ranging IFR and vaccine effectiveness against mortality in the two-dose scenario (first panel) and three-dose scenario (second panel). The y-axis shows deaths averted, in millions. The x-axis shows IFR. Dark red lines show baseline vaccine effectiveness (80% in two-dose scenario and 90% in three-dose scenario), and dark blue lines show high vaccine effectiveness (95% in two-dose scenario and 99% in three-dose scenario).
In a two-dose scenario, when IFR was 5/1000 and 5/10,000, the cost per death rate averted ranged from $7,200 to $71,700 respectively, while in a three-dose scenario, it ranged from $10,700 to $107,700 respectively. With the same parameter estimates, death averted ranged from 5 to 0.5 million in a two-dose scenario, and 4.7 million to 0.6 million in a three-dose scenario.
The researchers observed that in the condition of 100% Omicron infection in LICs/LMICs, with a three-dose scenario, base-case parameters resulted in averted cost-per-death at $115,000 with an aversion of 0.5 million deaths.
On varying IFR between 5/1000 and 5/10,000, the cost per death rate averted ranged from $20,900 to $230,000, with vaccine effectiveness against mortality of 99% and 90%, respectively. With the same parameter estimates, death averted ranged from 2.9 to 0.3 million, respectively.
To summarize, the findings of this study showed that scaling up of the global COVID-19 vaccination program, especially in LICs/LMICs will result in averting millions of deaths due to COVID-19. Hence, the researchers highlighted the need for investment in mass vaccination in the milieu of the value of a statistical life (VSL).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Model-based estimates of deaths averted and cost per life saved by scaling-up mRNA COVID-19 vaccination in low and lower-middle income countries in the COVID-19 Omicron variant era. Alexandra Savinkina, Alyssa Bilinksi, Meagan C Fitzpatrick, A David Paltiel, Zain Rizvi, Joshua A Salomon, Thomas Thornhill, Gregg S. Gonsalves.medRxiv 2022.02.08.22270465; https://doi.org/10.1101/2022.02.08.22270465, https://www.medrxiv.org/content/10.1101/2022.02.08.22270465v1
- Peer reviewed and published scientific report.
Savinkina, Alexandra, Alyssa Bilinski, Meagan Fitzpatrick, A. David Paltiel, Zain Rizvi, Joshua Salomon, Thomas Thornhill, and Gregg Gonsalves. 2022. “Estimating Deaths Averted and Cost per Life Saved by Scaling up MRNA COVID-19 Vaccination in Low-Income and Lower-Middle-Income Countries in the COVID-19 Omicron Variant Era: A Modelling Study.” BMJ Open 12 (9): e061752. https://doi.org/10.1136/bmjopen-2022-061752. https://bmjopen.bmj.com/content/12/9/e061752.
Article Revisions
- May 11 2023 - The preprint preliminary research paper that this article was based upon was accepted for publication in a peer-reviewed Scientific Journal. This article was edited accordingly to include a link to the final peer-reviewed paper, now shown in the sources section.