While the majority of infections with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will recover, a significant minority progress to severe or fatal coronavirus disease 2019 (COVID-19). A new study published on the preprint server medRxiv* investigates the role played by neutrophil extracellular traps (NETs) formation and degradation in COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
COVID-19 is considered primarily a pulmonary disease; however, it has also been associated with acute respiratory distress syndrome, multiorgan dysfunction, thrombotic disease of the microvasculature with tissue hypoxia, and skin manifestations of inflammation. In children, a multi-system inflammatory syndrome in children (MIS-C) has been reported to occur in some patients following COVID-19.
Chilblain-like lesions (CLL) have also been reported, mostly in younger people. Otherwise referred to as “COVID toes,” this presentation will include swelling, redness, or bluish discoloration of the fingers and toes, with vesicles in some cases. CLL is associated with lymphocytic infiltration of the skin glands and blood vessels in the affected area and appears to be independent of the severity of COVID-19.
While the incidence of CLL rose significantly during the current pandemic, many patients tested negative for the virus by polymerase chain reaction (PCR) while also being seronegative for SARS-CoV-2-specific antibodies. These lesions are known to be associated with high concentrations of type I interferons (IFNs), and the increased production of IFN-alpha in this patient population appears to indicate rapid clearance of the virus, thus explaining the lack of test positivity.
Earlier research indicates that SARS-CoV-2 infection triggers excessive NET formation, which is central in the manifestations of the disease. NETs are formed by the extrusion of neutrophil chromatin bound to granule proteins and are formed during inflammation, which can be due to infection or other health conditions.
NETosis can trigger vascular injury by initiating the transition from endothelial to mesenchymal tissues, causing endothelial and smooth muscle cells of the vessel walls to die. This leads to the activation of the coagulation cascade.
While severe COVID-19 has been associated with NET complexes by blocking small vessels in the kidneys and lungs, the respective rates of NET formation and breakdown, as well as the exact role of NETs in the pathogenesis of COVID-19 remain unclear.
The current study aimed to determine whether NETosis was present in children with either MIS-C or CLL at higher rates as compared to adults hospitalized with COVID-19. The mechanisms leading to the impaired breakdown of NETs in some COVID-19 patients were also explored.
Study findings
NET remnants were found to be present at higher levels in both MIS-C and CLL, though the levels varied between locations and with specific disease symptoms. In the United States and Italy, pediatric CLL samples showed higher levels of NET remnants than those from healthy controls.
Similarly, Chilean samples from children with MIS-C showed higher NET remnants but not those from Italy. Interestingly, all lesion samples from CLL children in the U.S. showed NETs, mostly around the blood vessels and beneath the skin.
In Chilean MIS-C samples, the extent of NET remnants was related to a greater likelihood of requiring cardiovascular drugs, developing pulmonary complications, or going into shock. Taken together, it appears that NETosis is associated with specific forms of disease following SARS-CoV-2 infection that are related to the severity of the disease, and may also underlie tissue damage in children with CLL.
The breakdown of NETs appears to be reduced in children with CLL and MIS-C but with a distinctive geographic variation. In samples from Italy, the majority of MIS-C samples and less than a fifth of CLL samples showed persistence of NETs beyond the expected time frame. Conversely, in the Chilean MIS-C samples, as well as both CLL and MIS-C samples from the U.S., 97% and 100% showed a reduction in the breakdown of NETs, respectively.
Even the rate of breakdown differed, being higher in Italian than Chilean MIS-C samples, or than U.S. CLL samples. This could be corrected by the addition of DNase1 in most cases, though between 25% and 30% of both sample types continued to show significantly impaired degradation. Therefore, some patients appear to have DNase1 inhibitors or other inhibitory mechanisms that prevent nuclease activity.
Similarly, NET breakdown was impaired in over half of adult COVID-19 patient samples from Italy, mostly if they were symptomatic at the time of diagnosis. This was reduced significantly by three months.
Increased production or reduced breakdown of NETs may be linked to a higher probability of organ damage in COVID-19. In the current study, the spleen, kidneys, and lungs showed the most extensive pattern of NET infiltrates.
Complement activation may also be associated with poor NET breakdown. Anti-NET autoantibodies have been found in adult COVID-19 patients. These autoantibodies, as well as the natural inhibitor molecule G-actin, are also implicated in a poor NET breakdown.
Adult COVID-19 patients with symptomatic illness also exhibit a persistent rise in NET remnants as compared to those with asymptomatic illness, which can persist even three months after infection at equivalent levels. This may correlate with the presence of long Covid symptoms months after the first diagnosis.
Moreover, higher NET remnant levels were associated with more severe disease, especially citrullinated H3-DNA, but not elastase-DNA, which showed varying patterns. The presence of these remnants was more likely with underlying cardiovascular/chronic kidney disease, or solid organ transplantation. The former was associated with liver disease, and the latter with congestive heart failure.
NET infiltrates were more commonly and extensively found in the spleen, kidneys, and lungs, and less commonly in the liver. In half of these cases, NETs were identified in cardiac tissue. These results show that NETs occur throughout the body in severe COVID-19.
As compared to other variants, SARS-CoV-2 Omicron infection is associated with lower levels of NETs, especially in males. Even in those with critical disease with hypertension or on high-flow oxygen and in the intensive care unit (ICU), this difference persisted, showing the variation in NET dysregulation with virus variant.
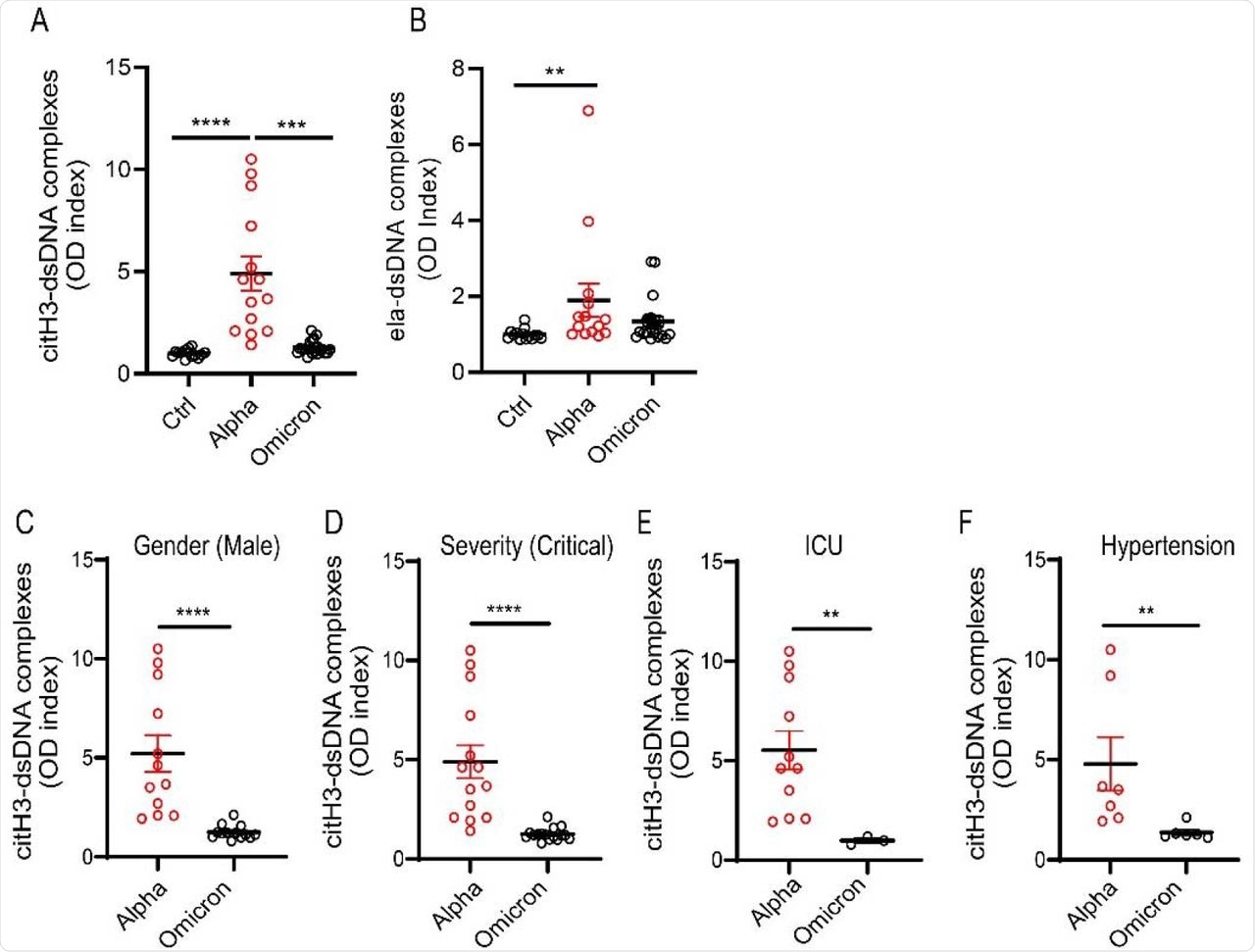
NET remnants are lower in adult unvaccinated patients infected with the Omicron variant. Plasma levels of (A) citrullinated histone H3 (citH3) and (B) elastase-DNA complexes (ela-dsDNA) were measured in COVID-19 patients infected with SARS-CoV-2 Alpha or Omicron variants (ctrl n= 14, Alpha n=14, Omicron= 21). Kruskal-Wallis analysis was used. (C) Male patients infected with the alpha variant of SARS-CoV-2 displayed elevated levels of citH3-DNA complexes (Alpha, n=12, Omicron, n=13). (D) Patients with critical severity of disease have lower levels of NETs when infected with Omicron infected patients displayed decreased levels of plasma citH3-DNA complexes (Alpha n= 14, Omicron n= 16). (E) COVID-19 patients in the intensive care unit (ICU) displayed decreased elevated levels of citH3-DNA complexes when infected with Omicron variant (Alpha, n=11, Omicron n= 3) (F) COVID-19 patients with concomitant hypertension displayed decreased elevated levels of citH3-DNA complexes when infected with Omicron variant (Alpha n= 7, Omicron n= 6), Mann-Whitney was used. Results are the mean +/- SEM. Mann-Whitney was used. **p<0.01, ***p<0.001, ****p<0.0001. OD: optical density.
Implications
While no genetic factors were found to affect NET formation in this study, other factors were identified that could change the neutrophil response to infection and/or inflammation. The period of follow-up was important in ruling out short-term autoimmune changes; however, considering the geographic differences, further assessments of the MIS-C groups from Chile and Italy will be necessary.
The current study suggests that NETs could be linked to severe or symptomatic COVID-19, other than by direct neutrophil infection. The persistent elevation of NETs indicates an ongoing dysregulation, which should be further examined for association with long Covid symptoms or other long-term complications.
The lower NETs observed with Omicron infection could be due to its lower propensity to elicit NET formation or due to the lower levels of inflammation it induces. Further studies may help uncover the role of NETs in tissue damage outside the lung that leads to severe forms of COVID-19 and its long-term complications.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Carmona-Rivera, C., Zhang, Y., Dobbs, K., et al. (2022). Multicenter Analysis of Neutrophil Extracellular Trap Dysregulation in Adult and Pediatric COVID-19. medRxiv. doi:10.1101/2022.02.24.22271475. https://www.medrxiv.org/content/10.1101/2022.02.24.22271475v1.
- Peer reviewed and published scientific report.
Carmona-Rivera, Carmelo, Yu Zhang, Kerry Dobbs, Tovah E. Markowitz, Clifton L. Dalgard, Andrew J. Oler, Dillon R. Claybaugh, et al. n.d. “Multicenter Analysis of Neutrophil Extracellular Trap Dysregulation in Adult and Pediatric COVID-19.” JCI Insight 7 (16): e160332. https://doi.org/10.1172/jci.insight.160332. https://insight.jci.org/articles/view/160332.