The coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) vaccines are marginally effective in neutralizing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs). Thus, there remains a need to generate next-generation COVID-19 vaccines that can neutralize VOCs and variants of interest (VOIs).
Study: Broad neutralization of SARS-CoV-2 variants by circular mRNA producing VFLIP-X spike in mice. Image Credit: Design_Cells / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Current mRNA vaccine design
The SARS-CoV-2 spike protein has been used as the primary target of current COVID-19 mRNA vaccines. Moreover, the SARS-CoV-2 spike protein consists of two proline substitutions (S-2P) in the receptor-binding domain (RBD), which makes it a potent immunogen that is capable of eliciting a good immune response.
However, SARS-CoV-2 has evolved with multiple mutations in the RBD region to give rise to several variants. The current RBD-specific neutralizing antibodies that have been generated in response to mRNA vaccination are not potent in neutralizing emerging variants.
Even so, neutralizing activity can still be elicited against the spike protein, particularly RBD, thus making the RBD a good candidate for vaccine design. Moreover, the SARS-CoV-2 RBD can be strategically engineered to elicit potent immune responses against several SARS-CoV-2 variants.
Next-generation vaccine design
Recently, researchers have designed a novel spike protein known as the five (V) prolines, Flexibly-Linked, InterProtomer disulfide (VFLIP). VLIPconsists of five proline substitutions in the S2 subunit of the spike protein, a flexible S1/S2 linker, and two cysteine substitutions that form an inter-protomer disulfide bond.
The VFLIP spike has improved thermostability while maintaining native RBD motions and glycosylation patterns that are similar to SARS-CoV-2. Mice immunized with VFLIP subunit candidate vaccine elicit a superior immune response as compared to the original S-2P-based vaccine; thus, this antigen design strategy can help elicit a broader immune response.
COVID-19 mRNA vaccines rely on linear mRNA molecules that are inherently unstable, which is comparable to circRNAs, which remain stable because of their covalently closed structure. The circularization of RNAs also protects these molecules from degradation by enzymes and allows for durable protein expression.
VFIP-X in a circRNA platform
In the current study, researchers designed a spike protein VFLIP-X that contained six rationally substituted amino acids and was subsequently incorporated in the circRNA platform. The selected mutations were observed in several VOCs and VOIs.
The investigators first confirmed that the circRNA containing firefly luciferase was capable of protein expression when injected intramuscularly in mice. The mice showed bioluminescence 24 and 48 hours after injection, which confirmed that the circRNA template was suitable for the development of an mRNA vaccine prototype.
The researchers then confirmed that the VFLIP-X spike could be expressed as a full-length protein. To this end, HEK293T cells were transfected with a circRNA prototype encoding the spike protein, VFLIP-X spike, or HexaPro-X, which is a spike protein with six amino acid substitutions. Using three different techniques including western blotting, immunofluorescence staining, and flow cytometry analyses with a polyclonal anti-RBD antibody, HexaPro-X and VFLIP-X both caused similar expression of full-length proteins.
Thirdly, the investigators assessed if VFIP-X conferred immunity against SARS-CoV-2. The circRNAs expressing HexaPro-X or VFLIP-X were formulated with lipid nanoparticles and injected into mice intramuscularly as two doses separated by a three-week interval. Only VFLIP-X induced high levels of neutralizing antibodies against B.1.1.529 pseudovirus.
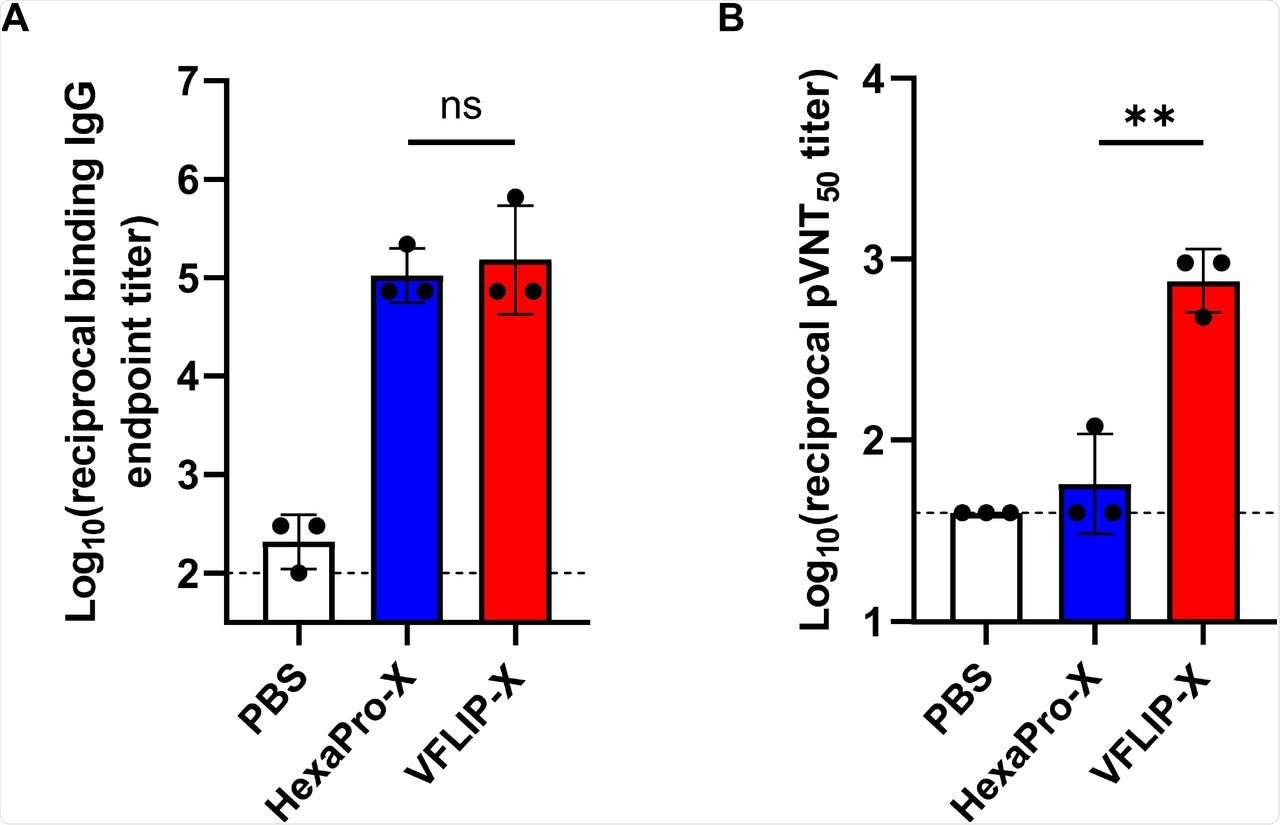
Mice immunized with VFLIP-X full length spike produce neutralizing antibody against B.1.1.529. BALB/c mice (n=3) were immunized at weeks 0 and 3 with 5 μg of circRNA-LNP encoding HexaPro-X (blue) or VFLIP-X (red). Control mice were administered with PBS (white). (A) IgG levels of two weeks post-boost sera were assessed by enzyme-linked immunosorbent assay (ELISA) using recombinant Omicron (B.1.1.529) spike protein. (B) Neutralization activity of two weeks post-boost sera were determined using B.1.1.529 pseudotyped virus. Data are presented as GMT ± geometric SD. Horizontal dotted lines represent assay limits of detection. Immunized groups were compared by student’s t-test (parametric, two-tailed unpaired). ** p < 0.01.
The researchers were also interested in determining whether VFLIP-X was capable of eliciting cross-neutralizing antibodies against several SAR-CoV-2 variants. To this end, blood sera samples from mice seven weeks after the booster dose were analyzed for neutralizing activity against live SARS-CoV-2 variants including the wild-type, B.1.1.7, B.1.351, B.1.617.2, and B.1.1.529 strains, as well as B.1, B.1.429, B.1.621, C.37, and B.1.1.529 pseudoviruses. This study revealed that VFLIP-X exhibited enhanced neutralizing activity against B.1.1.529, the SARS-CoV-2 variant with a large number of mutations, and strong neutralizing activity against other strains, VOCs, and VOIs.
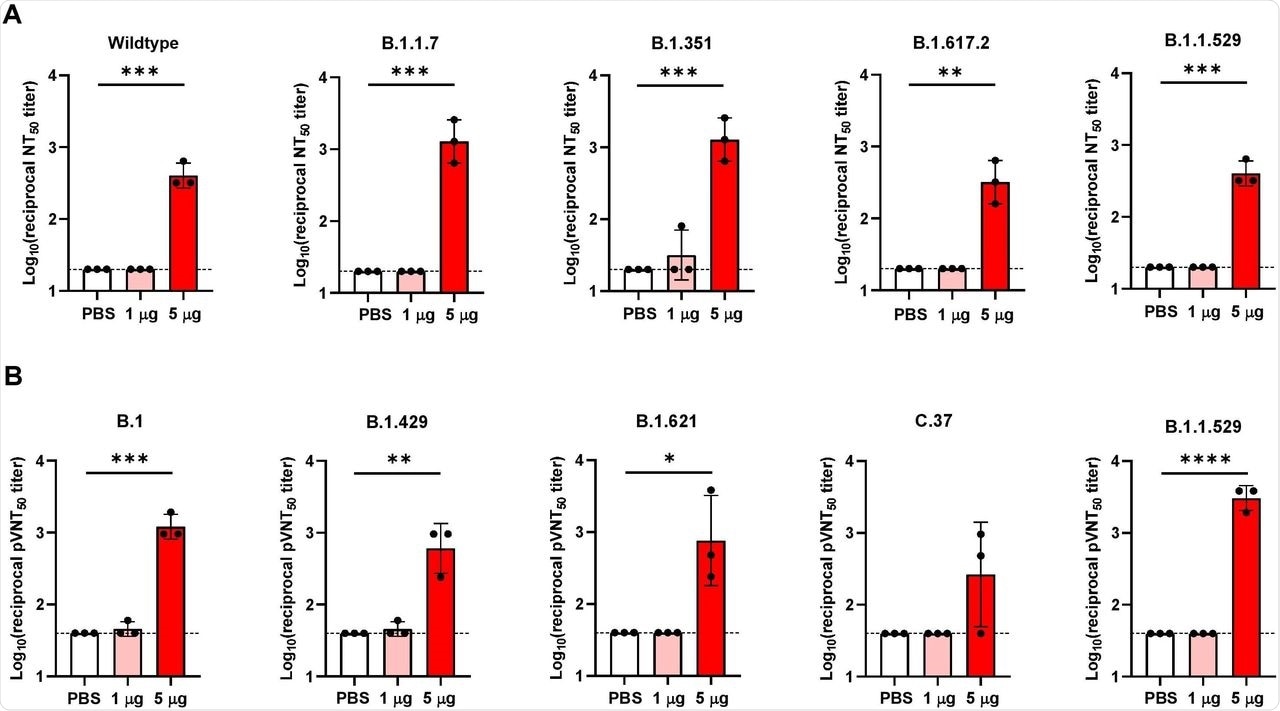
VFLIP-X elicits cross-neutralizing antibodies against SARS-CoV-2 VOCs and VOIs. BALB/c mice (n=3) were immunized at weeks 0 and 3 with 1 μg (pink) or 5 μg (red) of circRNA-LNP encoding VFLIP-X. Control mice were administered with PBS (white). (A) Live virus 50% neutralization titers (NT50) of seven weeks post-boost sera were determined against the indicated SARS-CoV-2 variants. (B) Lentivirus-based pseudovirus 50% neutralization titers (pVNT50) of seven weeks post-boost sera were determined against the indicated SARS-CoV-2 variants. Data are presented as GMT ± geometric SD. Horizontal dotted lines represent assay limits of detection. Control group was compared with 5 μg immunized group by student’s t-test (parametric, two-tailed unpaired). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Lastly, the investigators assessed the humoral and cellular immune responses elicited by VFLIP-X against the B.1.1.529 variant. VFLIP-X induced high levels B.1.1.529 S-binding antibodies after two doses of immunization that were dose-dependent and sustained up to seven weeks after the booster dose.
After the booster dose, neutralizing antibody levels increased at five weeks and sustained up to seven weeks. Likewise, cellular responses against the B.1.1.529 spike were also observed in mice seven weeks after the booster dose.
Conclusions
The current study demonstrates the potential of the circRNA vaccine prototype producing VFLIP-X to elicit neutralizing antibodies for up to seven weeks after the booster dose against SARS-CoV-2 VOCs and VOIs. This vaccine prototype also induced favorable immune responses, both humoral and cellular.
Taken together, these findings support future testing of circRNA next-generation COVID-19 vaccines for protection against emerging SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Seephetdee, C., Bhukhai, K., Buasri, N., et al. (2022). Broad neutralization of SARS-CoV-2 variants by circular mRNA producing VFLIP-X spike in mice. bioRxiv. doi:10.1101/2022.03.17.484759. https://www.biorxiv.org/content/10.1101/2022.03.17.484759v1
- Peer reviewed and published scientific report.
Seephetdee, Chotiwat, Kanit Bhukhai, Nattawut Buasri, Puttipatch Leelukkanaveera, Pat Lerdwattanasombat, Suwimon Manopwisedjaroen, Nut Phueakphud, et al. 2022. “A Circular MRNA Vaccine Prototype Producing VFLIP-X Spike Confers a Broad Neutralization of SARS-CoV-2 Variants by Mouse Sera.” Antiviral Research 204 (August): 105370. https://doi.org/10.1016/j.antiviral.2022.105370. https://www.sciencedirect.com/science/article/pii/S0166354222001395.