Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), scientists have been conducting extensive studies to understand various aspects of the virus. Researchers have stated that SARS-CoV-2 manifests several biological and clinical consequences of a superantigen. Therefore, a new study published in the journal Pathogens has focussed on SARS-CoV-2 as a superantigen.
 Perspective: Superantigens and SARS-CoV-2. Image Credit: Corona Borealis Studio / Shutterstock
Perspective: Superantigens and SARS-CoV-2. Image Credit: Corona Borealis Studio / Shutterstock
Superantigen: A Brief Overview
Superantigens are proteins that hyper-stimulate immune responses. These proteins may trigger T cells via the crosslinking of T cell receptors (TCR) with MHC Class II molecules or even hyper-stimulating B cells without the crosslink. Researchers have defined superantigens as a molecule associated with antigen-receptor mediated interactions with over 5% of the lymphocyte pool. A single superantigen can induce a wide array of host responses.
Hyperstimulation of T cells may lead to several outcomes, such as inflammation, cytotoxicity, deletion of T-cells, and autoimmunity. Previous studies have also reported that superantigens can impair post-vaccination memory cell responses to unrelated antigens and antagonize the activation of the memory cell. Individuals who express specific MHC Class II haplotypes that can bind with superantigens, experience toxic shocks. However, those individuals who express MHC Class II haplotypes with lower binding affinity do not experience such a shock. Some studies have reported superantigen-like proteins can trigger thrombotic and bleeding complications via platelet activation. Various factors may influence superantigen’s response, for example, simultaneous bacterial and viral infections. These affect the functions of the central nervous system and develop cardiovascular dysfunction and neurological conditions.
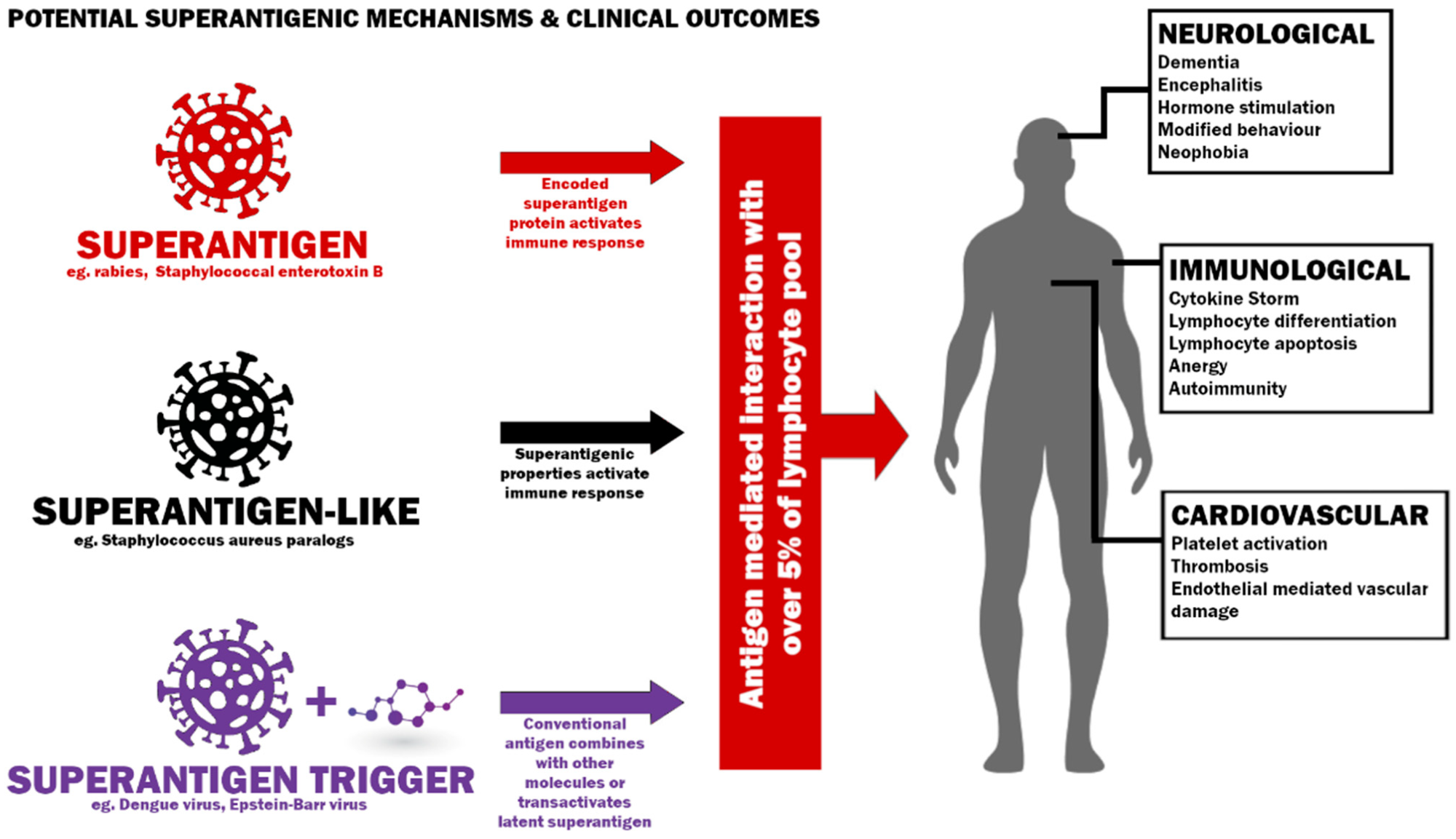 Potential mechanisms to induce a superantigenic host response and possible clinical outcomes.
Potential mechanisms to induce a superantigenic host response and possible clinical outcomes.
In the context of Dengue Virus (DENV) infection, activation of T lymphocytes influences the pathogenesis of dengue hemorrhagic fever (DHF). Interestingly, researchers stated that some of the clinical characteristics caused by DENV, such as T cell activation, autoimmunity, and neurological complications, are similar to COVID-19 infection. However, a study associated with TCR Vβ gene usage in children with DENV infection revealed that dengue is not a superantigen, but a conventional antigen. Interestingly, another study has revealed that conventional antigens can trigger a superantigenic host response. In addition, previous studies have shown that human endogenous retroviruses (HERV) contain proteins that act as superantigens.
Superantigens and Immune Responses
Scientists revealed that superantigens show a differential effect on immature and mature CD4 and CD8 T-cells. It can reduce thymocytes or immature T-cells as well as hyper-stimulate mature, antigen-experienced CD4s and CD8s. Scientists revealed that post hyperstimulation by Staphylococcal enterotoxin B (SEB) superantigen, T-cells fail to respond, i.e., it enters a state of unresponsiveness which is known as anergy, and subsequently, the cell undergoes apoptosis. Additionally, superantigens influence the differentiation of naive T-cells. They also trigger CD8 memory cells by activating cytokines or Vβ gene segments in their TCRs. Chronic exposure to superantigen could persistently stimulate T-cells, keeping them in a constant state between anergy and hyperstimulation. Additionally, naive T-cells are not readily produced in this condition due to thymic involution. Such conditions have been observed in some Long COVID patients. Depletion of naive T-cells occurs during immune aging and dysfunction.
Several studies have reported that superantigens cause autoimmune diseases. Superantigens trigger autoantibody generation by bridging the MHC Class II molecule of B-cells with the TCR on T-cells. As a result, individuals with autoimmune diseases exhibit enhanced T-cells in affected organs or peripheral blood.
SARS-CoV-2 and Superantigen
A recent study reported that individuals infected by SARS-CoV-2 with mild to moderate symptoms and who experienced Long COVID revealed depletion of naive T and B-cells. Some of the key factors that surfaced in previous studies, such as post-SARS-CoV-2 autoantibodies, activation, depletion of T-cells, and presentation of MIS-C, have indicated SARS-CoV-2 to be a superantigen, superantigen-like protein, or a causative agent triggering a superantigenic host response. In the future, more studies are required to elucidate its role and long-term effects, as this virus can persist for a prolonged period after acute infection.
T-cells carrying the TRBV11-2 gene with variable alpha chains have been identified as a signature hallmark of superantigen-mediated T-cell activation in patients with MIS-C. To date, it is not clear if SARS-CoV-2 is a superantigen; however, recent evidence definitely indicates it to be one. Similar to the Kawasaki disease, which is caused due to superantigen exposure, SARS-CoV-2 manifests similar conditions that include cytokine storms, T-cell activation, deletion, and the presence of MIS-C. Lipopolysaccharide (LPS) can trigger the SEB superantigen effect, on T cells following gut inflammation or injury via LPS translocation. Many studies have also indicated that SARS-CoV-2 infects gut epithelial cells and damages tight junctions in bronchial epithelial barriers. Additionally, patients who did not survive after COVID-19 infection revealed increased LPS in blood. A reduction in MIS-C following COVID-19 vaccination supports the preventive role of antibodies in the clinical manifestation of a superantigen or superantigen-like infection.