What is pyroptosis?
Pyroptosis is a proinflammatory type of cell death that is associated with gasdermin-mediated membrane hole formation, as well as cell enlargement and lysis. Pyroptosis plays an important role in both infectious and non-infectious diseases, some of which include atherosclerosis, autoimmune diseases, acute injury, adverse pregnancy events, cancer, and neuronal diseases.
Several pathogen-associated molecular patterns (PAMPs) and microbe-associate molecular patterns (MAMPs) occur prior to pyroptosis, thus contributing to the formation of inflammasomes, as well as the activation of caspases 1 and 11. The NOD-, LRR, and pyrin domain-containing protein 3 (NLRP3) inflammasome is one of the most notable upstream events of pyroptosis.
An overview of GSDMD
Following the activation of innate immune receptors by PAMPs, NLRP3 proteins accumulate in primed cells until ultimately undergoing conformational changes and oligomerization. These events lead to the recruitment of caspase 1, which subsequently cleaves full-length GSDMD that is responsible for the release of IL-1 and IL-18 proforms.
Of the five types of gasdermins, GSDMD is considered to be the most important pyroptosis effector molecule in myeloid cells including macrophages, monocytes, and dendritic cells. More specifically, GSDMD is primarily responsible for pore formation during pyroptosis, as it is essential for the release of proinflammatory ILs, the flux of ions, as well as the trafficking of nucleotides.
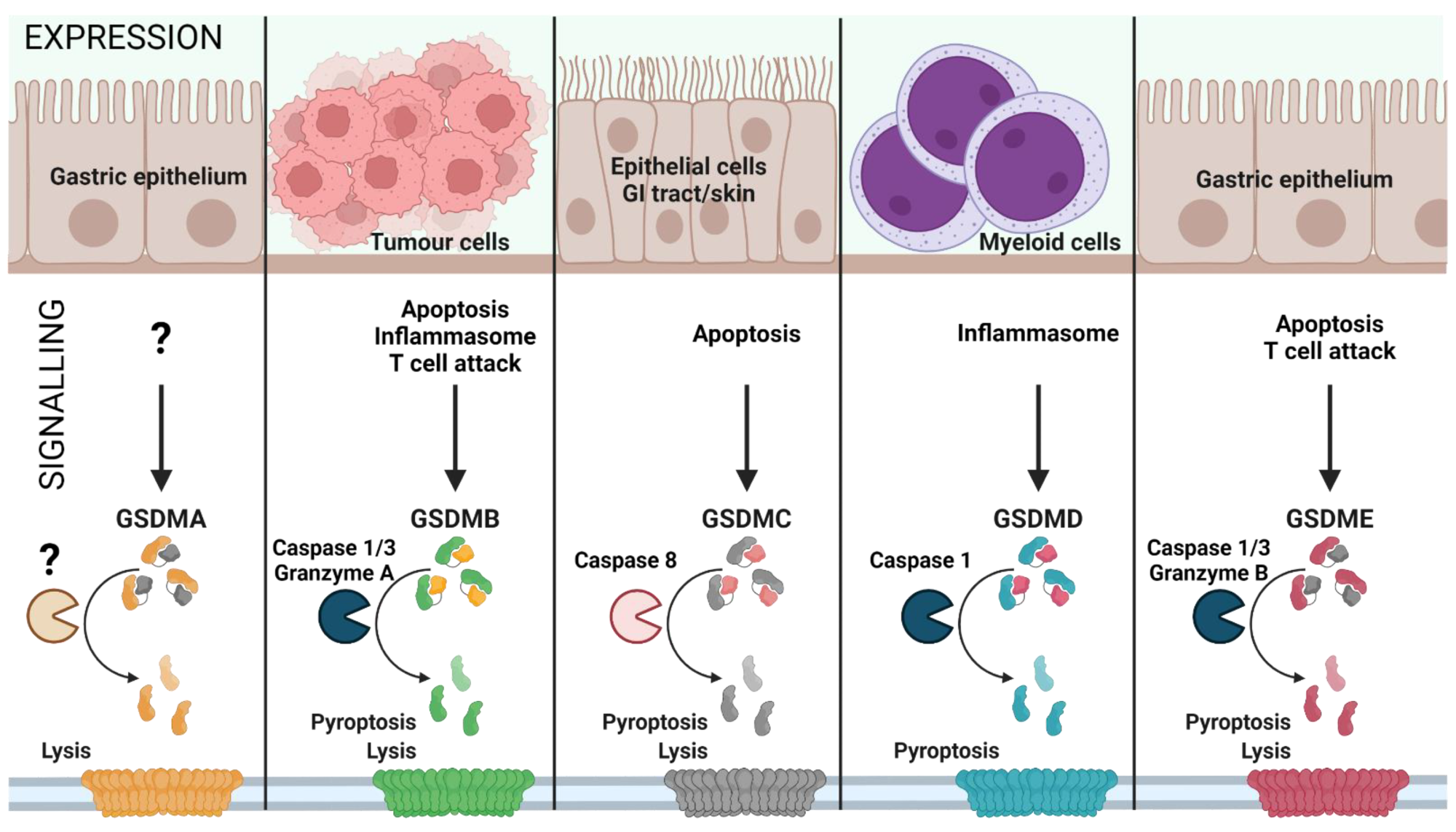
Gasdermin family: tissue-specific signaling. Gasdermin A/B/C/D/E are expressed in distinct cell types and are activated by various signals, leading to inflammatory or non-inflammatory cell lysis. The upstream events of GSDMA cleavage are not well characterized (represented as ‘?’). GSDMA downregulation in gastric epithelial cells can lead to tumor formation. Proliferating tumor cells are recognized by effector T cells that release Granzyme A, which cleaves GSDMB in cancer cells leading to pore formation and tumor cell lysis. Furthermore, GSDMB can also be activated by Caspase 1 or 3 downstream of inflammasome formation or apoptosis, leading to pyroptosis. GSDMC is activated by Caspase 8 downstream of apoptosis, linking apoptosis to pyroptosis. GSDMD is the best characterized GSDM effector molecule, cleaved downstream of inflammasome activation, leading to pyroptosis. Similar to GSDMC, GSDME links apoptosis to pyroptosis subsequent to cleavage by Caspase 3 in apoptotic cells. (Created with BioRender.com on 7 May 2022).
The role of NLRP3 in SARS-CoV-2 infection
Understanding the link between SARS-CoV-2 infection and pyroptosis-mediated inflammation has been crucial since the start of the pandemic. In fact, many researchers have been interested in exploring how inflammasomes and pyroptosis-mediated inflammatory signatures may offer a therapeutic target for the coronavirus disease 2019 (COVID-19).
Several in vitro and in vivo studies have revealed that the SARS-CoV-2 spike and envelope proteins prime the NLRP3 inflammasome. Furthermore, SARS-CoV-2 infection upregulates the expression of the angiotensin-converting enzyme 2 (ACE-2) receptor, which subsequently initiates nuclear factor κB (NF-κB)-mediated NLRP3 gene transcription and translation.
Previous studies have demonstrated that the SARS-CoV open reading frame 3 (ORF3) protein could directly bind to tumor necrosis factor-receptor associated factor (TRAF) and subsequently active NLRP3. This led researchers to also investigate whether the SARS-CoV-2 ORF3 protein exhibited similar activation of NLRP3, which was ultimately confirmed.
In addition to ORF3, the SARS-CoV-2 ORF8b directly interacts with the NLRP3 leucin-rich repeat domain (LRR), ultimately leading to pyroptosis. Both the SARS-CoV-2 nucleocapsid (N) and nonstructural protein 6 (NSP6) proteins have also been identified as pyroptosis initiators.
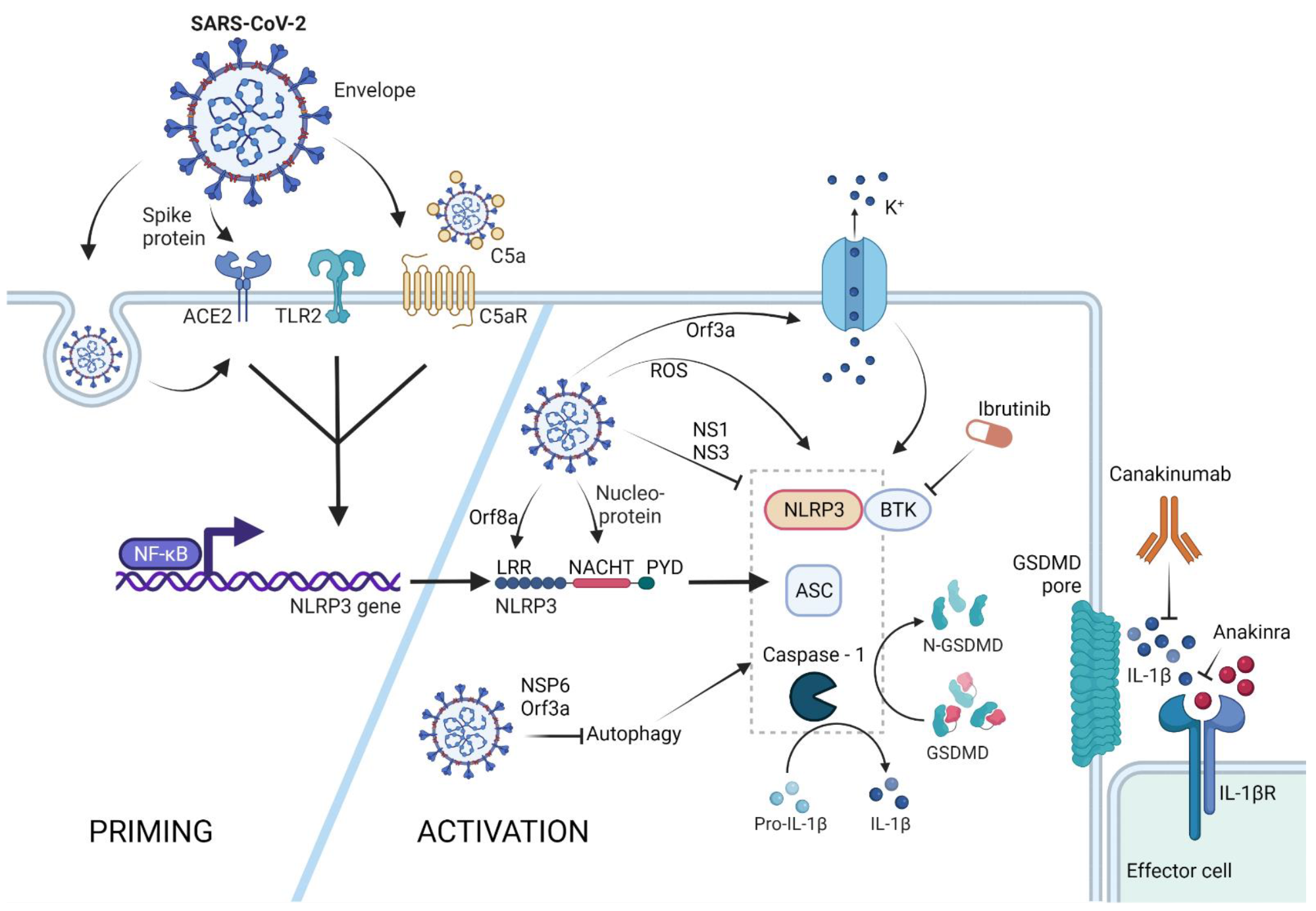
NLRP3 inflammasome activation by SARS-CoV-2. The NLRP3 inflammasome can be primed by SARS-CoV-2 proteins spike and envelope, and in its opsonized form by activating the ACE-2, TLR-2, and complement receptors, respectively. SARS-CoV-2-infected cells upregulate ACE-2 receptor expression, rendering these cells more sensitive to SARS-CoV-2 response. Activation of these receptors leads to NFκB-mediated Nlrp3 gene transcription and translation. The NLRP3 inflammasome can form in response to ROS generation in SARS-CoV-2-infected cells, via potassium efflux through SARS-CoV-2 Orf3a-generated pores, or via direct interaction with the SARS-CoV-2 proteins orf8a and nucleoprotein. SARS-CoV-2 is capable of NLRP3 inflammasome inhibition via NS1 and NS13 proteins, as well as through inhibition of autophagy, a general NLRP3 trigger. Therapeutic potential lies in the inhibition of the effector molecule IL-1β via canakinumab or anakinra, as well as inhibition of the NLRP3 accessory protein BTK by ibrutinib. (Created with BioRender.com on 13 April 2022).
How NLRP3 predicts COVID-19 symptoms and severity
Some patients infected with SARS-CoV-2 have reported experiencing a wide range of neurological symptoms. One recent study found that the SARS-CoV-2 spike protein directly interacts with the ACE-2 receptor in human microglia, which then leads to the activation of NLRP3 inflammasomes. This NLRP3-mediated pyroptosis in microglia may be responsible for the neuroinflammation that has been reported in a subset of COVID-19 patients.
The activation of NLRP3 has also been observed in COVID-19 patients and has been associated with COVID-19 severity. Thus, NLRP3 activation could be a useful predictor of disease severity, as well as a possible therapeutic target.
Notably, several biomolecules that are downstream of NLRP3 have been used as targets to treat COVID-19, particularly in patients experiencing severe forms of the disease. Some of these include canakinumab, which is an anti-IL-1β antibody, as well as anakinra, which blocks the IL-1 receptor.
More recently, researchers have identified the potential utility of ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor, which is an NLRP3-specific regulatory protein. In fact, the use of acalabrutinib, which is an ibrutinib-like BTK inhibitor, to treat severe COVID-19 patients was found to significantly increase the patients’ lung function.
Implications
Recent studies have indicated that COVID-19 is an inflammatory condition that could likely be treated through the inhibition of pyroptosis. Several agents targeting both upstream and downstream biomolecules of pyroptosis have been assessed for their ability to treat and assist in the diagnosis of COVID-19. Nevertheless, further research is needed to fully evaluate the utility of these agents against COVID-19.
Journal reference:
- Bittner, Z. A., Schrader, M., George, S. E., & Amann, R. (2022). Pyroptosis and Its Role in SARS-CoV-2 Infection. Cells. doi:10.3390/cells11101717.