The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the rapid outbreak of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has significantly affected the global healthcare system and economy. Several studies have reported that individuals of older age, as well as those with certain comorbidities like obesity and diabetes, are at a higher risk of contracting severe SARS-CoV-2 infection.
Background
Although most COVID-19 patients suffer mild to moderate symptoms, 10-15% develop severe pneumonia-like symptoms. In some individuals, COVID-19 causes tissue damage and triggers metabolic abnormalities, which can include the abnormal synthesis of lipids, insulin, and amino acids. The underlying molecular mechanism associated with these metabolic anomalies has yet to be determined.
Previous studies have revealed that the insulin/insulin-like growth factor 1 (IGF) signaling pathway has an important role in energy metabolism. Other molecular pathways associated with energy uptake and utilization are the downstream AKT/mTOR/MAPK pathway and ligand/receptor interactions that initiate cellular signaling through IRS/PI3K.
Insulin resistance or abnormal insulin/IGF responses occurs due to impairment in the insulin/IGF signaling pathway in organs and tissues. In addition to insulin resistance, an impaired insulin/IGF signaling pathway also leads to hyperglycemia, diabetes, hyperlipidemia, and obesity.
One previous study showed that the systemic knockout of the insulin receptor in mice initiated an early onset of diabetes. Additionally, some mice died due to ketoacidosis.
Inhibition of the insulin/IGF signaling pathway in adipose tissue results in the loss of adipose tissue. This condition also causes severe metabolic syndrome, thus demonstrating that insulin/IGF signaling is vital for the functioning of pancreatic beta cells.
Previous studies have also demonstrated that knockout of the insulin receptor in beta cells caused a reduction in insulin production, a condition otherwise known as diabetes mellitus. The association between insulin resistance and lung dysfunction in humans has also been documented in the literature. To date, very few studies have determined the impact of SARS-CoV-2 infection on the insulin/IGF signaling pathway.
About the study
In a recent Metabolism study, scientists evaluate the impact of SARS-CoV-2 on the insulin/IGF signaling pathway. Herein, the researchers obtained high-throughput transcriptome data related to SARS-CoV-2 infected cells and tissues from public databases.
The researchers also obtained autopsy data on the lungs of both COVID-19 and non-COVID-19 control patients. They further assessed the impact of SARS-CoV-2 infection on human pluripotent stem cell (hPSC)-derived liver organoids.
The current study evaluated the impact of IRF1 on insulin signaling using HEK293T and Calu3 cells. To evaluate the role of COVID-19 risk factors such as sex, age, obesity, and diabetes on mechanistic regulation, the scientists analyzed several transcriptomes of human metabolic, respiratory, and endocrine cells and tissues.
Study findings
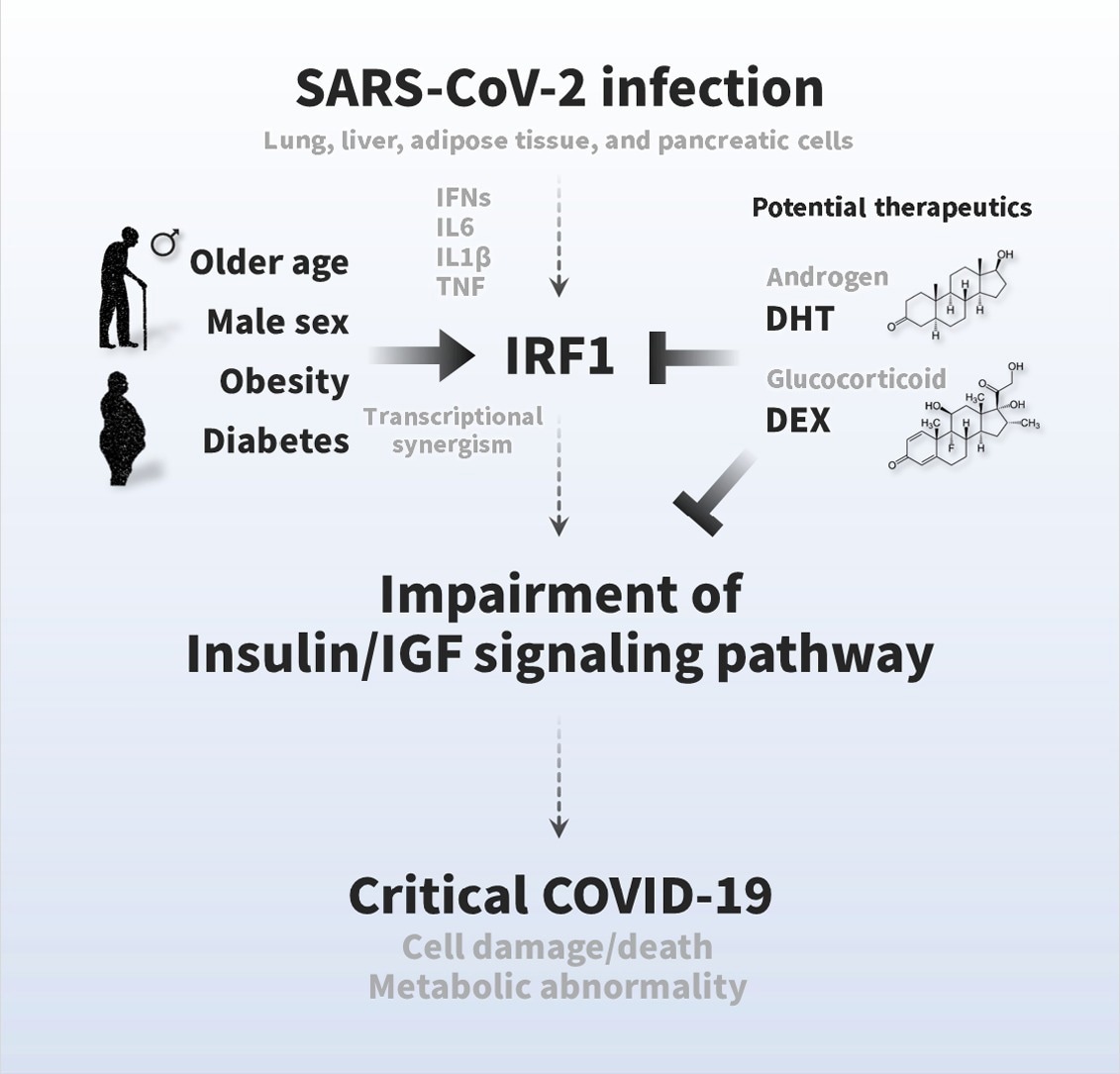
A strong association between an impaired insulin/IGF signaling pathway and severe COVID-19 was observed. Moreover, the researchers found that SARS-CoV-2 infection affects a wide range of insulin/IGF signaling pathways in the lungs, liver, pancreatic cells, and adipose tissue.
IRF1 was also found to be associated with the impairment of the insulin/IGF signaling pathway. Previous studies have linked the expression of IRF1 with male sex, older age, diabetes, and COVID-19. In fact, a higher expression of IRF1 has been associated with obesity, older age, diabetes, and/or genetic variant, which might be intensified due to the upregulation of IRF1 in response to SARS-CoV-2 infection.
Previous studies have shown that IRF1 intron variant rs17622656-A is abundantly present in European and American populations. The current study reports that COVID-19 pathology is governed by the higher expression of IRF1 and lower expression of insulin/IGF signaling pathway molecules.
Hormonal treatment in the form of dihydrotestosterone and dexamethasone has effectively reduced the gene expression of IRF1. These findings led the researchers to hypothesize that this treatment could increase the insulin/IGF signaling pathway in SARS-CoV-2 infected cells and tissues and, as a result, improve cellular signaling.
This finding strongly indicates that SARS-CoV-2 infection not only affects respiratory tracts but also impairs endocrine and metabolic cells and tissues. Identifying the molecular basis of these pathological observations could therefore aid in the development of future COVID-19 therapeutics.
Impairment of the insulin/IGF signaling pathway in cells and tissues of the liver, lungs, and pancreas leads to the development of multiorgan dysfunction. Since COVID-19 causes impairment of the insulin/IGF signaling pathway, it is also associated with the downregulation of various metabolic pathways including the citrate cycle, lipid metabolism, beta-oxidation, amino acid metabolism, and carbohydrate metabolism, and respiratory electron transport.
The researchers also emphasized the potential for COVID-19-mediated inflammation to induce insulin resistance in various cells and tissues.
Conclusions
The current study showed that dihydrotestosterone and dexamethasone treatments could ameliorate insulin resistance and metabolic abnormalities in COVID-19 patients. In the future, more physiological, pathological, and pharmacological experiments must be conducted to estimate the clinical efficacy of this treatment in COVID-19 patients.
Journal reference:
- Shin, J., Toyoda, S., Nishitani, S., et al. (2022) SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 133. doi:10.1016/j.metabol.2022.155236