Although COVID-19 is predominantly a respiratory disease, the clinical outcomes of severe SARS-CoV-2 infections have been seen to affect multiple organ systems. Endothelial dysfunction, micro thrombosis, and hypercoagulation associated with myocarditis-like presentations of impaired cardiac function have been observed in 20% to 30% of hospitalized COVID-19 cases. Furthermore, vascular remodeling and microthrombi formation are associated with increased cardiac serum markers, severe disease, and mortality.
Despite the rising number of COVID-19 cases with clinical outcomes involving cardiac complications, the mechanisms of cardiac pathology and heart injury during severe SARS-CoV-2 infections remain unclear. Current hypotheses include myocardial and vascular wall damage due to cytokine storm, which is the increased release of proinflammatory cytokines such as interleukins (IL) 1 and 6, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), and the presence of SARS-CoV-2 viral particles in cardiomyocytes and cardiac macrophages.
Since the cardiac symptoms observed in COVID-19 are similar to other viral myocarditis forms, comparing cardiac autopsy tissue from these diseases might shed light on the cardiac pathology mechanisms of COVID-19.
 Study: Inflammation and vascular remodeling in COVID-19 hearts. Image Credit: Kateryna Kon / Shutterstock
Study: Inflammation and vascular remodeling in COVID-19 hearts. Image Credit: Kateryna Kon / Shutterstock
About the study
In the present study, the team analyzed 24 cardiac autopsy tissue samples from SARS-CoV-2 mortality cases and compared them to the closest sex, age, and disease severity-matched archived heart tissue from 16 influenza H1N1 and eight non-H1N1 and non- SARS-CoV-2 related myocarditis mortalities. Heart tissue samples from nine non-infectious and non-inflammatory disease-related heart surgeries were used as control.
A significant number of cases in each of the groups had exhibited serum markers indicating cardiac involvement, such as troponin, creatine kinase myocardial band (CK-MB), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), left or right ventricular ejection fraction (LVEF/RVEF), and abnormalities in the echocardiograph.
A wide range of techniques was employed to analyze molecular and morphological changes in the heart tissue samples, including histopathology, multiplex immunohistochemistry, synchrotron radiation tomographic microscopy, microvascular corrosion casting, and gene expression analysis. SARS-CoV-2 spike and nucleocapsid protein were used for the immunohistochemistry analysis. Additionally, ribonucleic acid (RNA) fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) was used to detect SARS-CoV-2 RNA from formalin-fixed paraffin-embedded samples.
Results
The results indicated that none of the COVID-19 heart tissue samples could be diagnosed as viral myocarditis based on histopathology. However, compared to the heart tissue from influenza and non- SARS-CoV-2 myocarditis patients, the COVID-19 cardiac autopsy tissue showed an increase in the perivascular cluster of differentiation molecule 11b (CD11b)/angiopoietin-1 receptor (TIE2) presenting macrophages during multiplex immunohistochemistry.
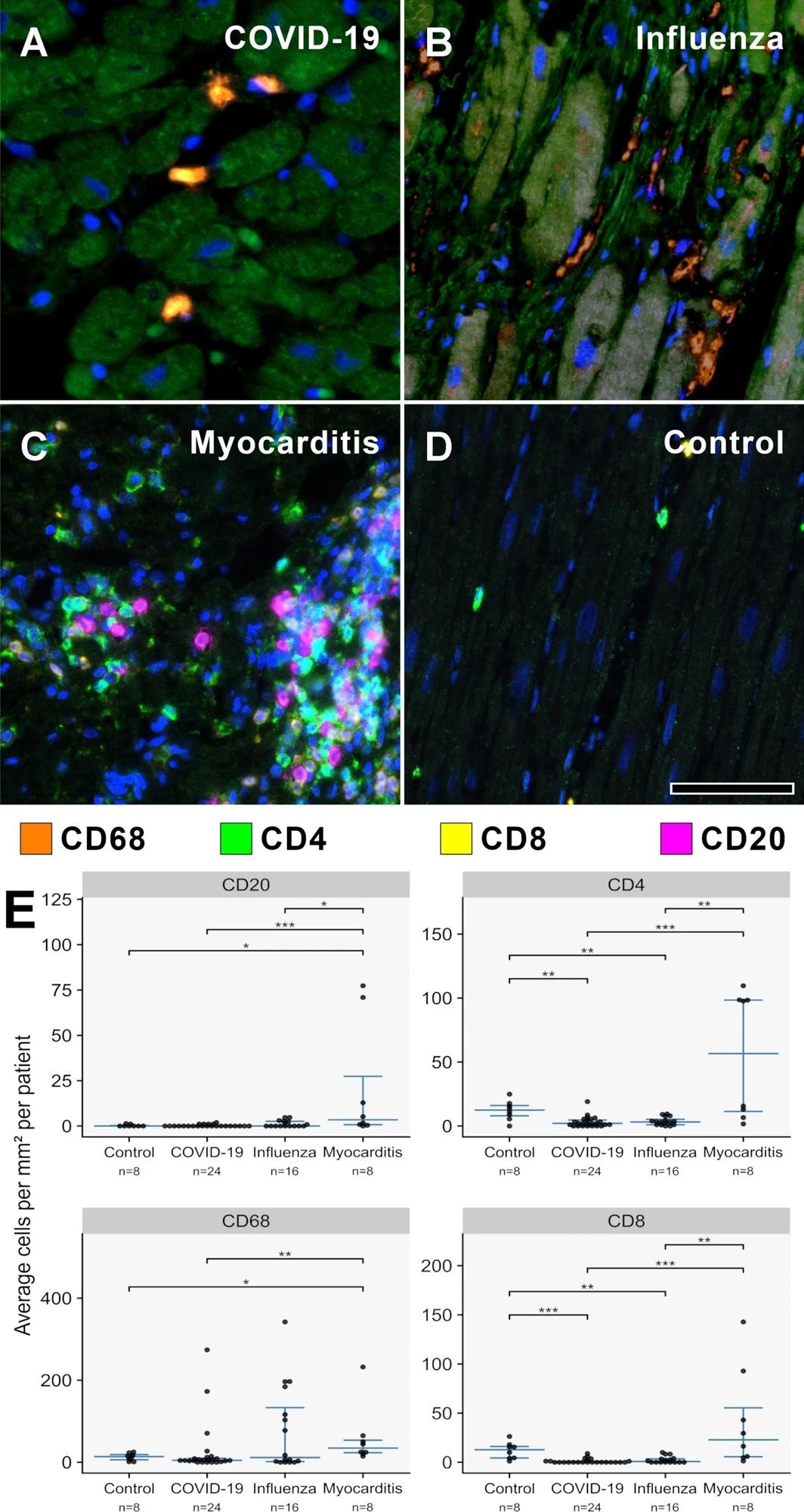
A–D MPX staining of cardiac tissue depicting CD68 + macrophages in orange, CD4 + T helper cells in green, CD8 + cytotoxic T cells in yellow, and CD20 + B-cells in magenta. All infected hearts (COVID-19, influenza, and lymphocytic non-influenza myocarditis) displayed a prominent infiltrate of CD68 + macrophages. While COVID-19 A (COVID-19 patient ID 24) and influenza, B (Influenza patient ID 9) hearts showed nearly absent lymphocytic infiltrate, lymphocytic non-influenza myocarditis, C (Myocarditis patient ID 5) was characterized by a mixed, T-cell dominated infiltrate. Non-infected control hearts, D (Control patient ID 1) showed markedly less inflammatory cells with a mixed population of macrophages and predominant t-cells and only scarce B-cells. Magnification 400x. Scale bars = 100 µm. E Histogram of the inflammatory cell infiltrates (CD20, CD4, CD68, CD8). Cell counts are normalized to cells per mm2 myocardial tissue. *p < 0.05, **p < 0.01, ***p < 0.001
Furthermore, heart tissue from COVID-19 patients also exhibited gene expression of epithelial-mesenchymal transition and angiogenesis factors, which was not observed in the cardiac tissue from the other groups. This supported the hypothesis that cytokine storm during COVID-19 is associated with endothelial damage and myocardial edema. In addition, increased intussusceptive angiogenesis and multifocal thrombi in COVID-19 cardiac tissue were identified as evidence of vascular remodeling. Other cardiovascular diseases such as atherosclerosis, viral myocarditis due to parvovirus B19, inflammatory diseases, and cancers also exhibit intussusceptive angiogenesis.
The stromal cell-derived factor 1/ C-X-C chemokine receptor type 4 axis (SDF-1/CXCR4) is thought to play an important role in myocardial repair during myocardial infarctions, viral myocarditis, and cardiomyopathies through the recruitment of bone-marrow-derived mononuclear cells. The increase in CD11b/TIE2+ macrophages, upregulation of SDF-1, CXCR4, and matrix metallopeptidase 9 (MMP9), and concurrent increase in intussusceptive angiogenesis seen in this study supported the role of the SDF-1/CXCR4 axis in myocardial repair.
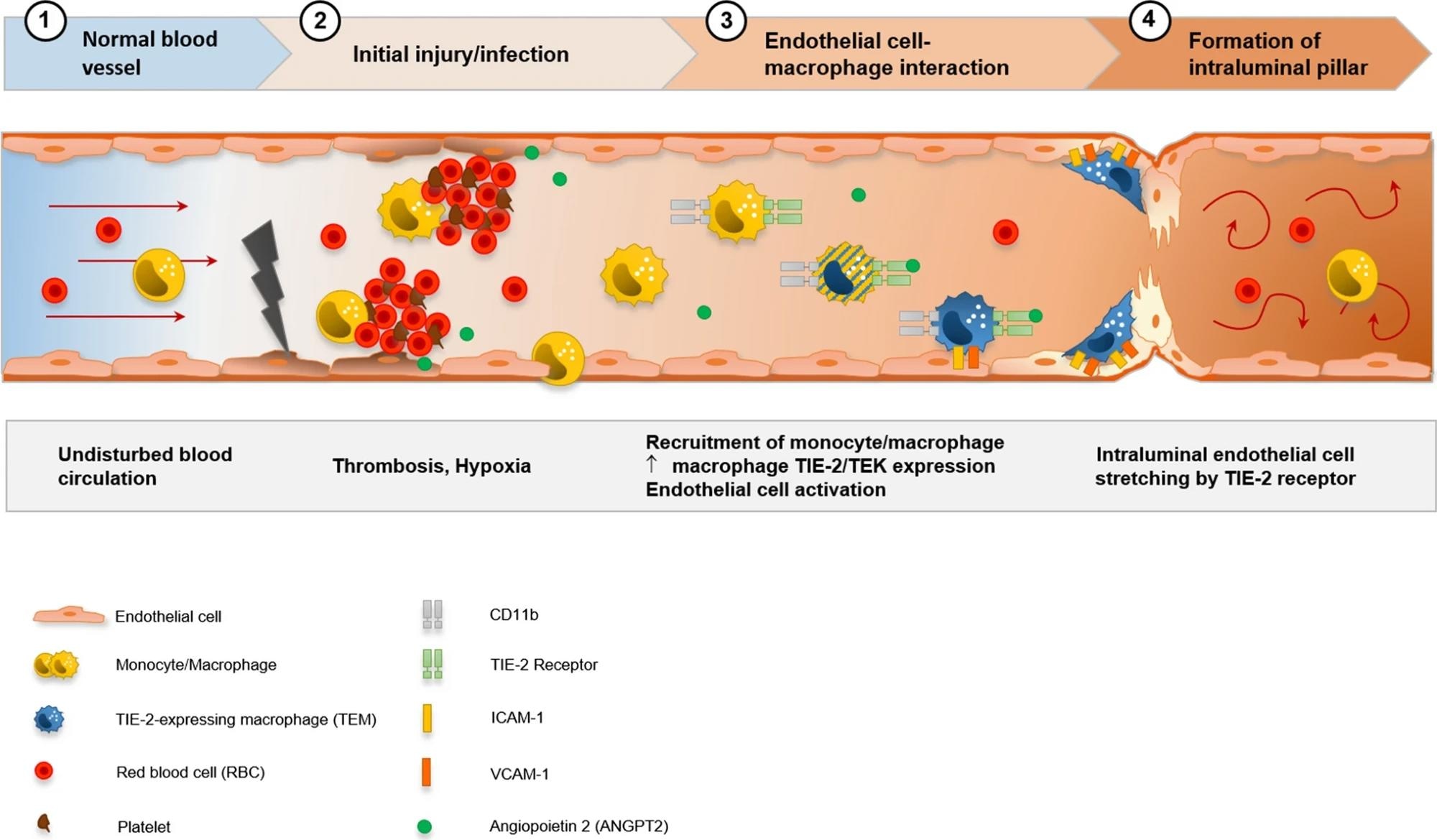 Visualization of the hypothesis of CD11b + /TIE2 + monocytes/macrophages recruitment and incorporation, and intussusceptive angiogenesis in the cardiac vasculature in COVID-19. SARS-CoV-2-related endothelial dysfunction results in thrombotic microangiopathy in cardiac capillaries and tissue hypoxia. Endothelial cells induce the recruitment of monocytes/macrophages to the site of injury by upregulation of adhesion-molecules and activation of SDF-1/CXCR4 signaling. TIE2 + monocytes/macrophages are activated by increased levels of angiopoietin 1 and adhere locally in response to angiopoietin 2 to endothelial cells. Formation of an intussusceptive pillar is achieved by intraluminal stretching of endothelial cells under the help of adherent TIE2 + monocytes/macrophages resulting in the division of a single capillary altering the cardiac microvasculature
Visualization of the hypothesis of CD11b + /TIE2 + monocytes/macrophages recruitment and incorporation, and intussusceptive angiogenesis in the cardiac vasculature in COVID-19. SARS-CoV-2-related endothelial dysfunction results in thrombotic microangiopathy in cardiac capillaries and tissue hypoxia. Endothelial cells induce the recruitment of monocytes/macrophages to the site of injury by upregulation of adhesion-molecules and activation of SDF-1/CXCR4 signaling. TIE2 + monocytes/macrophages are activated by increased levels of angiopoietin 1 and adhere locally in response to angiopoietin 2 to endothelial cells. Formation of an intussusceptive pillar is achieved by intraluminal stretching of endothelial cells under the help of adherent TIE2 + monocytes/macrophages resulting in the division of a single capillary altering the cardiac microvasculature
Conclusions
To summarize, the study investigated the mechanisms underlying the cardiac tissue damage associated with severe COVID-19 cases by comparing cardiac autopsy tissue from COVID-19 patients with those from influenza H1N1, non-viral myocarditis, and non-infectious and non-inflammatory cardiac surgery tissue.
Overall, the results suggested that COVID-19 cardiac tissue is not similar to conventional viral myocarditis heart tissue in terms of the infiltrates and damage observed. Furthermore, intussusceptive angiogenesis leading to irreversible vascular remodeling is one of the major causes of cardiac injury during severe COVID-19.
Journal reference:
- Werlein, C., Ackermann, M., Stark, H., Shah, H. R., Tzankov, A., Haslbauer, J. D., von Stillfried, S., Bülow, R. D., El-Armouche, A., Kuenzel, S., Robertus, J. L., Reichardt, M., Haverich, A., Höfer, A., Neubert, L., Plucinski, E., Braubach, P., Verleden, S., Salditt, T., & Marx, N. (2022). Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis. https://doi.org/10.1007/s10456-022-09860-7, https://link.springer.com/article/10.1007/s10456-022-09860-7