Studies report that the telomere sequence, TTAGGG, is constant across all vertebrates and serves a critical function in inhibiting the deoxyribonucleic acid (DNA) damage response by attaching to a group of proteins known as shelterin. Changes in telomere sequence decrease shelterin binding, cause DNA damage, and are toxic to cells.
 Study: A persistent variant telomere sequence in a human pedigree. Image Credit: Lightspring / Shutterstock
Study: A persistent variant telomere sequence in a human pedigree. Image Credit: Lightspring / Shutterstock
About the study
In the present study, researchers uncovered a novel heterozygous mutation in the TERC of an IPF patient that encodes a telomere sequence. This variation enabled the patient to remain illness-free for over four decades and was inherited.
Recently, researchers analyzed IPF patients and discovered some with uncommon mutations in TERC and TERT. An individual tested showed heterozygous-type C>A transversions in the TR template region. The medical history included premature graying and the diagnosis of idiopathic pulmonary fibrosis (IPF) at an early age of 43 years. To verify r.50C>A genetic variant-comprising telomerase activity in vivo, the researchers analyzed the proband's and his son's whole genome sequencing (WGS) results to investigate variant sequence integration into telomeres.
Using WGS information from peripheral blood mononuclear cells (PBMC from proband) or saliva (from the son) samples, researchers calculated the percentage of wild-type repetitions and mutants in a proband, a son, and ten controls. They used bespoke Perl programs to count wild-type and variant repetitions. They next tested variant telomere sequence identification in situ with PNA probes concerning three sequence variants or wild-type repeats. The proband in the study had undergone lung transplantation and investigations for wild-type telomeres and mutations using fluorescent in situ hybridization (FISH).
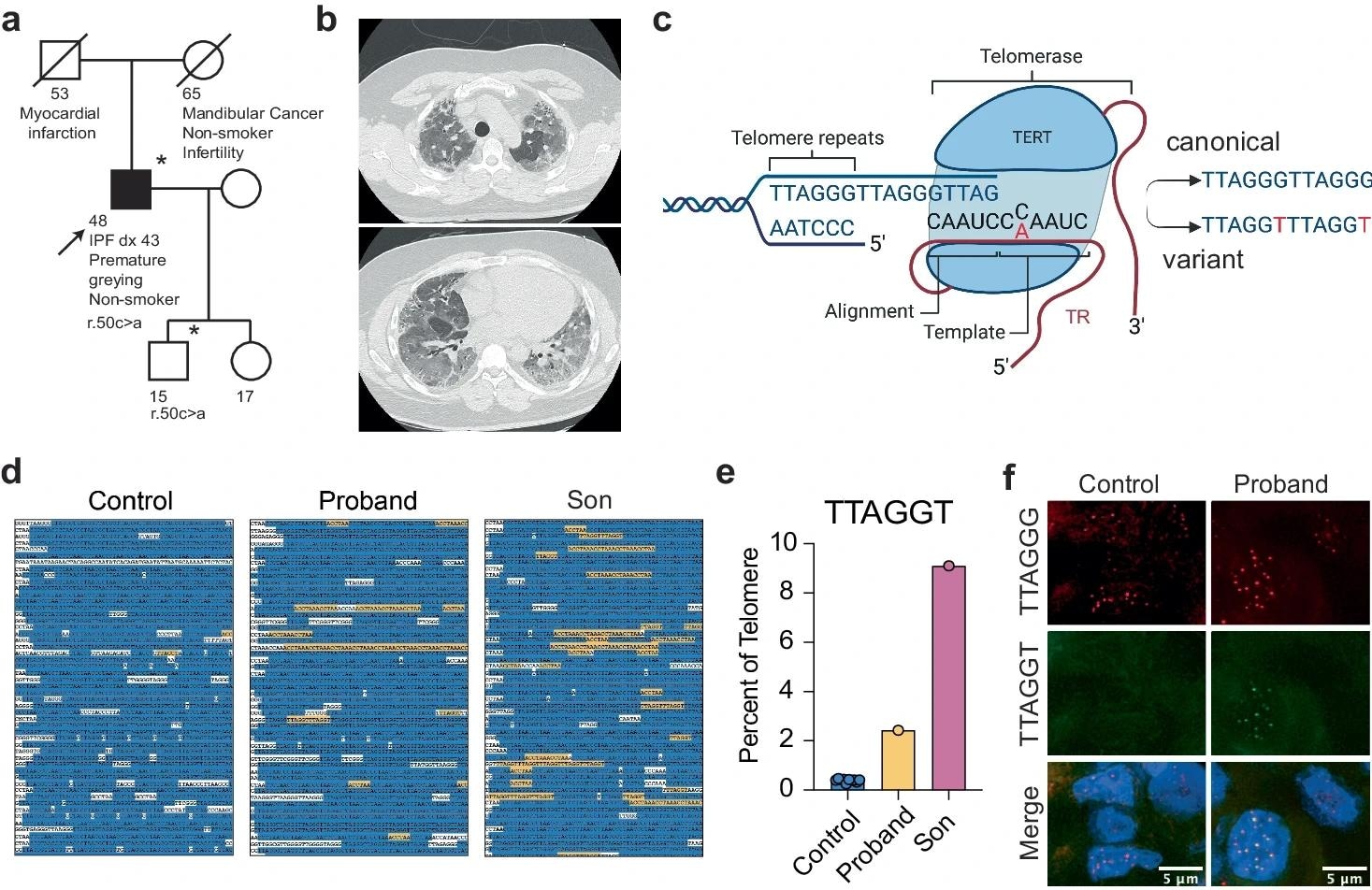 a Pedigree of the family carrying the TERC r.50 C > A variant. The proband (arrow) was diagnosed with IPF at age 43. Asterisks indicate individuals from which DNA was available. Squares indicate males and circles indicate females. A line through the symbol indicates the individual is deceased. b Apical (upper) and basal (lower) chest CT showing interstitial changes and advanced fibrosis in the proband before lung transplantation. c Graphic showing the location of the patient-derived variant in the template of TR and the resulting telomere sequence. Figure was created with BioRender.com and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 international license. d Excerpts of telomeric sequences from whole genome sequencing of an individual with no template mutation, the proband, and the proband’s son. Canonical repeats are highlighted in blue, variant TTAGGT repeats are highlighted in yellow, additional non-canonical sequences are in white. e The percentage of TTAGGT repeats in controls, the proband, and the proband’s son. f PNA-FISH for wild-type (red) and variant (green) sequence in a tissue section of the proband’s explanted lung and a control donated lung. Source data for (e) is provided as a Source Data file.
a Pedigree of the family carrying the TERC r.50 C > A variant. The proband (arrow) was diagnosed with IPF at age 43. Asterisks indicate individuals from which DNA was available. Squares indicate males and circles indicate females. A line through the symbol indicates the individual is deceased. b Apical (upper) and basal (lower) chest CT showing interstitial changes and advanced fibrosis in the proband before lung transplantation. c Graphic showing the location of the patient-derived variant in the template of TR and the resulting telomere sequence. Figure was created with BioRender.com and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 international license. d Excerpts of telomeric sequences from whole genome sequencing of an individual with no template mutation, the proband, and the proband’s son. Canonical repeats are highlighted in blue, variant TTAGGT repeats are highlighted in yellow, additional non-canonical sequences are in white. e The percentage of TTAGGT repeats in controls, the proband, and the proband’s son. f PNA-FISH for wild-type (red) and variant (green) sequence in a tissue section of the proband’s explanted lung and a control donated lung. Source data for (e) is provided as a Source Data file.
Researchers developed expression constructs for the variation TTAGGT sequence, which may result in deficient repeat addition processivity (RAP) due to a mismatch between TR at r.C56 and the 3' end of the mutant telomere. They also investigated whether POT1-TPP1, a recognized RAP booster, might recover the poor RAP in interactions with C50A.
The researchers investigated the dynamics and effects of introducing a variation sequence into TERT-positive cell lines, transducing cells with lentiviruses encoding several variants, and detecting telomere incorporation with peptide nucleic acid (PNA)-FISH probes. They also measured the telomere length of the study proband and analyzed the influence of variant telomerase on the 3' telomere terminal sequence. They also investigated whether POT1 might bind to the mutant sequence in vitro and block POT1-mediated suppression of telomerase activity.
Results
The analysis showed that a mutation in the telomerase gene carried one or more generations, and a family member reported non-significant medical issues despite almost 9.0% of the telomeres changing to the new sequence. The mutant template inhibited telomerase repeat-adding processivity and reduced POTR1 interactions. Despite these abnormalities, the sequence is easily integrated into cell chromosomes. The presence of variant sequences may affect telomere addition.
The proband child also bore the variation, although he reported no severe medical issues. The researchers expected the mutation r.50C>A to introduce the TTAGGT non-canonical-type telomere genetic sequence rather than TTAGGG. The proband telomeres contained 2.7% TTAGGT sequence while those of controls comprised 0.40% (6.0-fold higher in proband), and those of the proband’s son comprised 9.20% variant sequences, a 23.0-fold increase, indicating significant variant repeat additions into the germlines or developmental processes by telomerase.
The proband had constant canonical repetitions (84%), but his child had a lower amount (78%) than the controls. Approximately 15% of telomere fraction comprised neither TTAGGT nor wild-type sequences, comparable to earlier research that examined telomere composition using WGS data. Explanted lung tissues included the variable telomere sequence, but control donor lungs did not. Sequencing findings and in situ hybridization indicate that telomerase absorbs the sequences remaining in adult tissues.
Structural modeling demonstrated that a dG:rC pairing across this zipper is more energy stable than a dT:rA pairing. In hTERT-RPE cells, 36% of C50A- and 70% of C50/56A-transduced cells exhibited at least ten mutant telomere foci, with reduced contiguous TTAGGT repeats. The likelihood of processive addition by the variation TR in the proband and his child was 39% and 28%, respectively.
The study found that telomeres can withstand considerable degeneracy, implying that inserting a non-canonical telomere sequence may affect telomere length dynamics. Telomerase comprising C50A TR causes virtually total RAP loss due to mismatching between the budding mutant telomere and TR r.56C. Correcting the mismatch by producing C50/56A variations enhanced RAP, facilitating more POT1-TPP1-related activations. However, when overexpressed, C50/56A TRs lengthened telomeres just as much as wild-type TR. Mutated telomere sequences, like POT1ΔOB, may prevent shelterin from inhibiting telomerase addition.