Since the 1990s, Yokogawa has played a prominent role in the R&D of high-speed confocal microscopes for real-time imaging of living cells, and in 1996, launched its first product, the CSU10 confocal scanner unit. The company then released the CellVoyager™ high-throughput screening system, the CQ1 all-in-one image cytometer, the CellPathfinder cell image analysis software, and other advanced products. Yokogawa is a pioneer of live cell imaging technologies and drug discovery support systems.
In this interview, NewsMedical speaks with Kevin Jan from Yokogawa about the future of live cell imaging technologies.
The CSU10 was a landmark product for Yokogawa in 1996. How did its introduction impact the field of live cell imaging and Yokogawa's position in the market?
Using confocal microscopy, the CSU 10 enabled live imaging of cells and thick specimens through the z-axis with multipoint illumination and multipoint detection, whereas both the illumination and detection of conventional optical microscopy were planar. Also, using most of the confocal microscopes at the time, it typically took over a second to produce a single image. That was too slow to observe the movement of living cells in real-time.
The CSU10 provided scanning speeds as fast as 360 frames/second with a high signal-to-noise (S/N) ratio. Those characteristics allowed direct viewing of clear-cut, confocal images of fluorescent specimens in real-time at the eyepiece. The confocal images captured by the CSU10 could be recorded by a video camera as live images. Those allowed the study of real-time behavior and interactions among microorganisms. The imaging quality allowed a technician to record a clear-cut cross-section of a whole-mount embryo. Plus, the CSU10 could be mounted on most of the microscopes used at the time.
What were the key technological and market challenges Yokogawa faced while developing the CellVoyager™ high-throughput screening system?
The challenges were actually typical of a new, advanced technology. Aside from our collaboration—or, as we say, co-innovation—partners, many in the industry did not understand the technology or the possibilities it enabled. However, those involved in advanced drug discovery and development required what we referred to as “high content analysis” for phenotypic screening. The use of 3D samples, such as spheroids and organoids, and live cell samples to mimic realistic cellular environments was emerging as a key requirement due to cell culture technology development.
Assessing a very large number of compounds is time-consuming—but acquiring a large number of high-resolution cellular images with fast scanning speed is critically important to drug screening. The high-content analysis imagers balanced these generally conflicting requirements at a high level of performance. The core technology, the microlens-enhanced dual Nipkow disk in our confocal scanners and confocal spinning disc units (CSUs), makes it possible to capture high-quality images with remarkably short acquisition time.
A Nipkow spinning disk containing about 20,000 pinholes and a subsidiary spinning disk containing the same number of microlenses to focus excitation laser light into each corresponding pinhole are mechanically fixed on a motor and rotated at high speed. As a result, a high-speed raster scan of the excitation lights on the specimen can be achieved.
The pinhole and microlenses are arranged on each disk in a proprietary design to optimize the raster scan. Multi-beam scanning not only increases scanning speed but also results in significantly lower photobleaching and phototoxicity because multi-beam excitation needs only a low level of laser power on the specimen to fully excite fluorescence.
While there were technical paradigms to overcome, we were readily able to provide comparisons between high-content analysis (HCA) and other methods, such as flow cytometry, high-throughput screening, and traditional microscopy. Justifying the higher cost took time, but it ultimately hinged on helping prospective users understand the possibilities—and the significant competitive disadvantages of lagging in the fast-evolving drug discovery process. Moreover, early success stories provided crucial credibility.
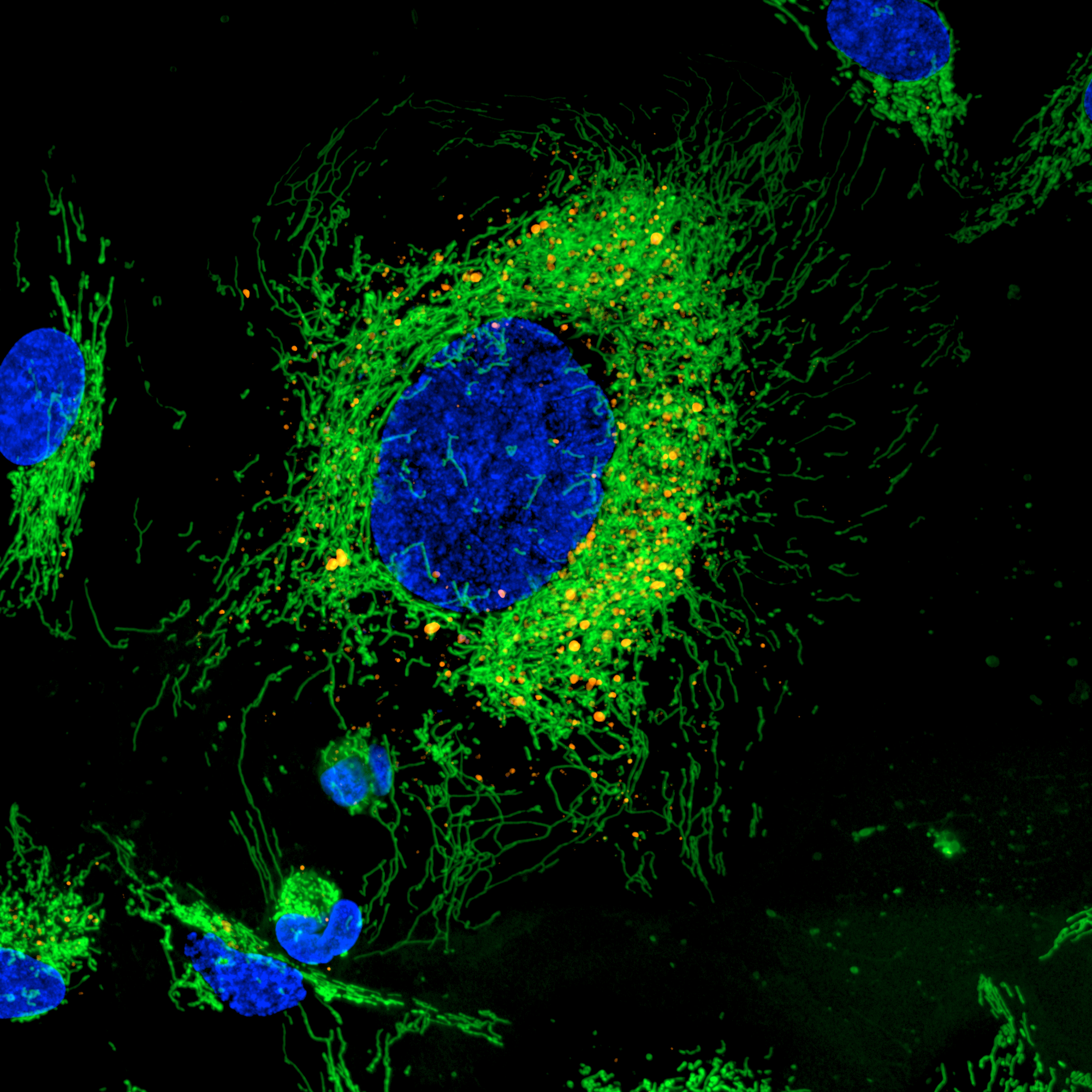
Image of Hela Cells taken by CV8000_MitoGreen (Green) and Dapi (Blue) 60x Water Immersion Lens, 50um Pinhole. Image Credit: Yokogawa Life Science
How does the CQ1 and CQ3000 image cytometer stand out in the competitive landscape of image cytometry, and what features make it particularly suited for drug discovery?
The CQ1 and CQ3000 are both benchtop units that combine the operability of a traditional flow cytometer with the high image quality of our HCA technology. They can be easily installed in any lab without the need for a darkroom or specialized setup. Despite its ease of use, the CQ1 and CQ3000 offer rich feature extractions for sophisticated cellular image analysis. Its Nipkow spinning disk confocal technology enables high-speed scanning while minimizing phototoxicity and photobleaching, significantly enhancing productivity.
The CQ1 and CQ3000 enable measurements of spheroids, colonies, and tissue sections. In contrast to traditional flow cytometry, cells are not removed from the culture dish. In addition, a high-precision stage incubator and low phototoxicity of the confocal make the analysis of time-lapse and live cells possible. It is also compatible with the CellPathfinder high-content analysis system software.
Could you shed light on the development journey of the CellPathfinder cell image analysis software and its contributions to the field of cellular analysis?
As advanced as the core, high content analysis hardware technology is, realizing the cellular imaging possibilities depends extensively on the analysis software. For instance, cell trace software machine learning functions dramatically increase target recognition and decrease slow turnarounds. Machine learning also continuously improves process success.
CellPathfinder provides abundant analysis functions to individualize research. It resolves screening bottlenecks and employs AI to simplify advanced analyses. It also provides detection of rare events (CTC, for example) with high speed and high accuracy.
We have also worked extensively with co-innovation partners to perfect the environment for specific applications such as colony formation, cytotoxicity, neurite outgrowth, co-culture analysis, and cell tracking.
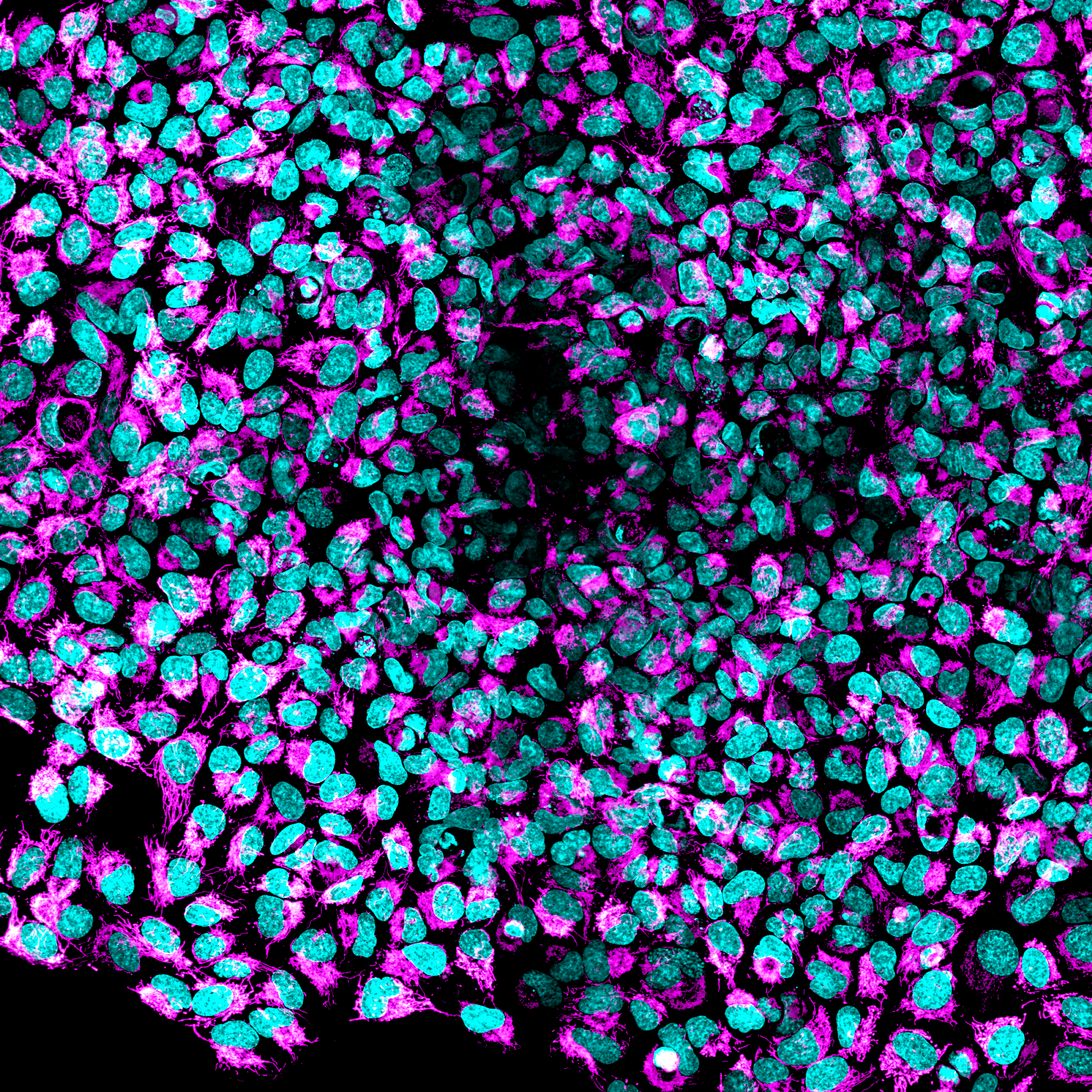
Image of HEK293 taken by the CQ1_50um pinhole. Image Credit: Yokogawa Life Science
What future advancements in live cell imaging technologies is Yokogawa currently focusing on or planning to explore?
We are working with co-innovation partners on new applications that can extend the capabilities of HCA technology. A number of proof-of-concepts are in process, and, in some cases, researchers have been able to report conclusions. For example, our latest single-cell analysis solution, the Single Cellome™ System, model SS2000, has been featured in an article published in the high-profile journal Analytical Chemistry regarding groundbreaking work conducted by researchers at the University of Surrey, England, in the emerging field of single-cell lipidomics.
The SS2000 is a live cell image device equipped with Yokogawa's original dual-microlens spinning disk imaging technology, enabling cutting-edge life science research.
Lipidomics is the large-scale study of lipids, a large family of molecules that play biological roles ranging from maintaining normal operation of the human body to the development of major diseases. Lipidomic studies are conventionally conducted by bulk isolation and analysis of lipids from many cells.
While bulk lipid analyses can provide scientists with a population average of the lipid profile, they are unable to discern subtle cell-to-cell variations or reveal the spatial or temporal differences in the lipidome caused by cell-cell interactions.
Single-cell lipidomics is an emerging field where the lipid composition of single cells is analyzed. These studies help overcome the challenges of bulk lipidomics by offering scientists a means of exploring spatial and temporal differences in addition to intercellular variability. Understanding these differences is key to creating a more complete picture to understand diseases such as cancer. One challenge facing researchers is the ability to isolate single cells in a way that maintains the natural lipidome of a cell.
Current methods of single-cell isolation detach and suspend multiple cells at once and then isolate them through a narrow channel, but this can be particularly stressful to cells and may result in alterations to cellular lipid make-up.
Yokogawa's SS2000 Single Cellome System uses confocal imaging technology to help overcome challenges with single-cell lipidomics. It is a dual-purpose system that enables live cell imaging and also performs fully automated single-cell and subcellular sampling without going through the suspension process, thereby minimizing stress on the cells.
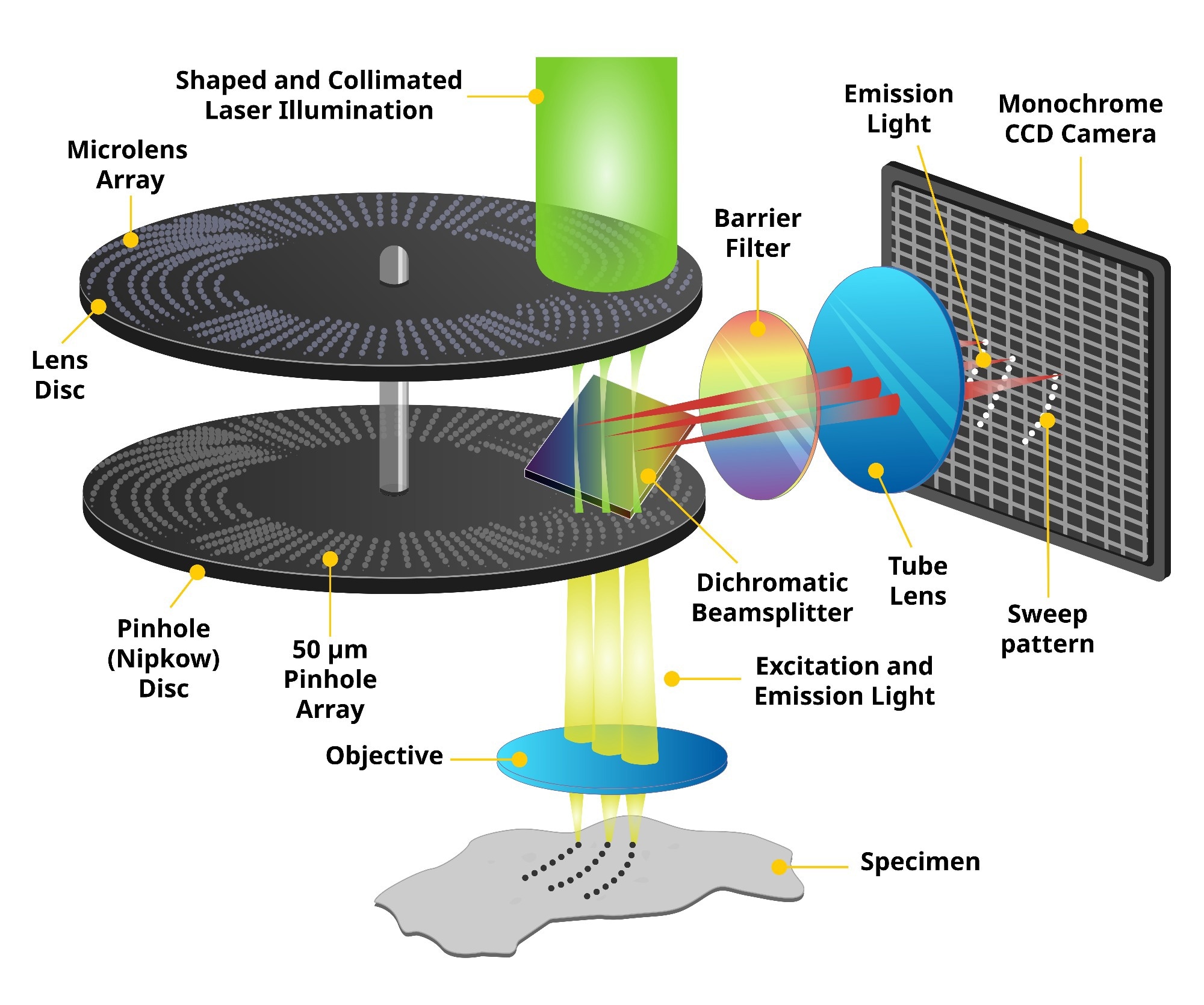
Spinning Disk Confocal Microscopy Technology. Image Credit: Yokogawa Life Science
How do partnerships and collaborations play a role in Yokogawa's strategy for developing and enhancing microscopy and imaging solutions?
Partnerships and collaborations with key stakeholders are integral to Yokogawa’s development process. The company uses the phrase “co-innovating tomorrow” as a corporate slogan. It represents the determination to co-create new value for solving customers’ problems and expanding new opportunities while cultivating long-term partnerships.
Yokogawa has also shifted the direction of the company’s R&D activities from not only developing new technologies and creating value on its own to co-creating new value in cooperation with customers.
The key idea is that we must abandon the conventional concept of company-centric value creation, establish a new paradigm of value co-creation between companies and customers, and radically change business activities to align with them. Furthermore, the concept of design thinking urges us not to simply derive ideas solely from technologies but to visit the field, observe users, and create an idea based on empathy.
How have industry conferences and forums influenced Yokogawa’s product development strategies and business relationships in the scientific community?
The conferences and forums have allowed us to expand our opportunities for co-innovation through exposure to new end-users, influencers, and industry leaders. In the life sciences industries, such events have been very active. In such a fast-changing industry, it is critical to interact on a nearly continuous basis.
How does customer feedback shape the evolution of Yokogawa's products, particularly in the field of confocal microscopy and image analysis?
While customer feedback is paramount in any industry, we see these fields as rapidly evolving and, thus, in need of even closer work in conjunction with customers. There is practically a continuous process of new developments in drug discovery and research. We take an approach that all customers are co-innovation partners.
About the Speaker

Kevin Jan currently holds the position of Principal at Yokogawa, where he leverages his extensive experience in process automation and control systems. With a strong background in engineering and a proven track record in the industry, Kevin plays a crucial role in driving innovative solutions and advancing Yokogawa's technological capabilities. His leadership and expertise contribute significantly to the company's success in delivering high-quality services to clients worldwide.
 About Yokogawa Life Science
About Yokogawa Life Science
We have 30 years of experience in this life science field and will respond to customer's problem solving with cutting-edge solutions.
Our confocal scanner unit CSU series enables 3D observation of the cells in detail and dynamics of organelles inside cells. Since the CSU series is capable of high-speed shooting, it is also suitable for observing high-speed life phenomena. In addition, the CSU series is a multi-point confocal method which is extremely gentle to cells, best suitable for long-term live cell observation.