On July 29, 2024, the U.S. FDA approved Alpha Cognition Inc.'s ALPHA-1062 (Zunveyl®), an Acetylcholinesterase (AChE) inhibitor, for treating mild-to-moderate Alzheimer’s disease. This condition affects approximately 6.7 million people in the United States. Over 70 % of physicians are dissatisfied with current therapies due to side effects and limited efficacy, with over 50 % of patients discontinuing treatment within a year. As research on AChEIs advances, the importance of ALPHA-1062 and similar molecules as therapeutic options for neurodegenerative disorders becomes increasingly evident.
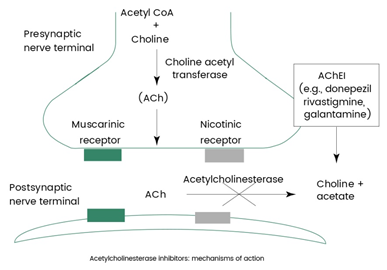
Image Credit: Sino Biological Inc.
Mechanism of action
Acetylcholinesterase (AChE) breaks down the neurotransmitter acetylcholine into choline and acetate at synaptic junctions. This process is crucial for terminating synaptic transmission, especially at neuromuscular junctions and cholinergic synapses in the nervous system. AChE's mechanism of action involves two critical sites: the anionic site and the esteratic site.
- Anionic Site: This site initially attracts and binds acetylcholine, facilitated by its positively charged quaternary amine, which interacts with the negatively charged environment of the anionic site.
- Esteratic Site: Following binding at the anionic site, acetylcholine is transferred to the esteratic site, where it undergoes hydrolysis. This site contains a catalytic triad composed of serine, histidine, and glutamate amino acids. The serine acts as a nucleophile, attacking the carbonyl carbon of acetylcholine, leading to the cleavage of the ester bond between acetyl and choline. This reaction results in the formation of acetate and choline.
The rapid hydrolysis of acetylcholine by AChE is essential for the regulation of neurotransmitter levels in the synaptic cleft, ensuring that nerve impulses are accurately passed on or halted as required. By controlling the duration of acetylcholine activity, AChE plays a vital role in muscle contraction, learning, memory, and other neurobiological processes.
AChE as drug target of Alzheimer's disease
In Alzheimer's disease management, ACE is targeted due to its role in rapidly breaking down acetylcholine, thereby influencing cognitive functions like memory and learning. Therapeutic strategies aim to increase acetylcholine concentrations, which can potentially enhance cognitive function and alleviate the symptoms of Alzheimer’s.
As a leading reagent supplier, Sino Biological offers recombinant AChE proteins which can be used to explore AChE's biological roles and interactions and help evaluate the impact of potential inhibitors. AChE-specific antibodies are another critical tool, enabling the detection and quantification of the enzyme in various tissues, which is crucial for understanding its pathological and physiological roles. In addition to AChE, Sino Biological offers other drug targets and biomarkers, such as Tau, BACE1, and ApoE, to fully support drug discovery and diagnostics development for Alzheimer's disease.