Background
Autism Spectrum Disorder (ASD) affects approximately 1 in 100 children worldwide, yet early diagnosis remains a challenge. Emerging research suggests that differences in white matter—the brain’s communication network—can be detected in infancy and may serve as early indicators of autism.
White matter develops rapidly during pregnancy and infancy, forming essential neural connections that support cognition and motor function. Genetic factors play a crucial role in this process, but their exact influence remains unclear.
Advances in neuroimaging now allow researchers to map these early brain changes, shedding light on how genetic predisposition shapes neural pathways. Understanding these links may lead to earlier interventions, improving outcomes for children at risk of autism.
While studies have explored white matter differences in older children, little is known about how genetic variants influence neonatal brain structure, necessitating further investigation.
About the Study
The present study analyzed white matter structures in 221 term-born infants of European ancestry from the Developing Human Connectome Project. Advanced diffusion-weighted imaging was used to capture high-resolution brain scans, allowing researchers to examine microscopic fiber density and macrostructural morphology. Data preprocessing included noise reduction, motion correction, and normalization to a study-specific brain template.
Genetic analysis involved saliva samples collected at birth or 18 months, which were processed to identify common genetic markers associated with autism. Quality control measures ensured data reliability, excluding samples with incomplete genetic information. Polygenic scores, representing cumulative autism risk, were calculated based on genome-wide association studies and adjusted for ancestry differences.
Statistical models assessed the relationship between genetic risk and white matter structure, accounting for variables such as total brain volume, gestational age, and sex. A gene-set enrichment analysis was conducted to identify biological pathways linked to white matter alterations associated with autism. Additional analyses were performed to explore whether specific genetic pathways influenced structural differences in white matter connectivity.
Study Results
Infants with higher autism polygenic scores showed a significant increase in fiber-bundle cross-section in the left superior corona radiata, a brain region crucial for motor and cognitive functions. This suggests that genetic predisposition to autism may shape early white matter organization, though further studies are needed to confirm its significance for later developmental outcomes.
Further analysis indicated that microscopic white matter properties remained unchanged, while macrostructural differences were prominent in the superior corona radiata and related tracts. These findings align with previous studies reporting increased white matter volume in infants and toddlers later diagnosed with autism. However, the study did not find significant microstructural differences, suggesting that the observed changes are more related to fiber bundle cross-section rather than density or organization at the microscopic level.
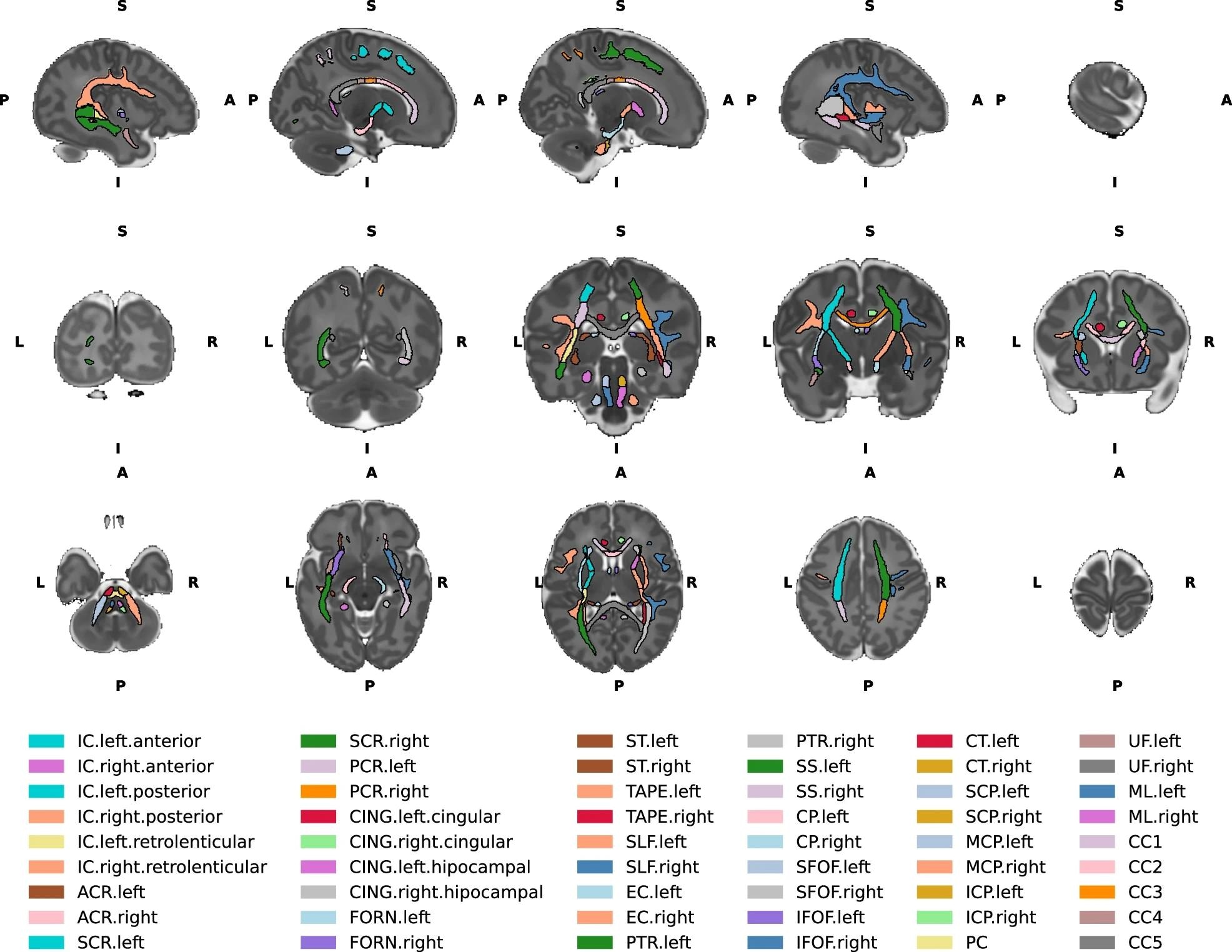
Top row - Left to right sagittal view of the brain slices. Middle row- Posterior to anterior coronal view of the brain slices. Bottom row - Inferior to superior axial view of the brain slices. IC internal capsule, ACR anterior corona radiata, SCR superior corona radiata, PCR posterior corona radiata, CING cingulum, FORN fornix, ST stria terminalis, SLF superior longitudinal fasciculus, EC external capsule, PTR posterior thalamic radiation, SS sagittal stratum, CP cerebral peduncle, SFOF superior fronto-occipital fasciculus, IFOF inferior fronto occipital fasciculus, CT corticospinal tract, SCP superior cerebellar peduncle, MCP middle cerebellar peduncle, ICP inferior cerebellar peduncle, PC pontine crossing, UF uncinate fasciculus, ML medial lemniscus, CC1 corpus callosum prefrontal part, CC2 premotor/supplementary motor part, CC3 corpus callosum motor part, CC4 corpus callosum sensory part, CC5 corpus callosum parietal/temporal/occipital part. L- left, R- right, A- anterior, P- posterior, S- superior, I- inferior.
A deeper investigation into brain connectivity patterns revealed that infants with higher autism polygenic scores had increased cross-sectional areas in additional white matter tracts, including pathways involved in sensorimotor and cognitive processing. These changes could play a role in the atypical brain connectivity observed in individuals with autism.
Genetic pathway analysis revealed that the autism-associated variants linked to white matter changes were overrepresented in genes related to neuronal connectivity and synaptic function. Notably, genes such as MAPT, KCNN2, and DSCAM—previously implicated in autism risk—were highlighted in the study, reinforcing the hypothesis that white matter alterations are linked to neurodevelopmental processes essential for cognitive and motor function.
While statistically significant, the effect sizes were small, and some findings—such as those related to the right superior corona radiata—did not survive multiple testing corrections, indicating the need for further validation.
These findings suggest that white matter alterations in neonates reflect genetic influences on early brain development rather than serving as definitive biomarkers for autism. If validated in larger studies, these results could have profound implications for early screening and intervention strategies, enabling proactive developmental support before behavioral symptoms emerge.
Conclusions
To summarize, these findings emphasize the profound impact of genetics on early brain development. By identifying structural brain differences at birth, researchers move closer to understanding autism’s earliest origins.
Detecting these alterations early could contribute to research on personalized interventions, allowing for targeted therapies before behavioral symptoms emerge. However, the study does not suggest that current neuroimaging techniques can reliably predict autism in neonates. As neuroscience advances, integrating genetic insights with neuroimaging may help predict neurodevelopmental outcomes, ultimately improving the lives of individuals with autism and their families.
Future research should explore how these early structural changes relate to long-term cognitive and behavioral development, shaping new strategies for early intervention and support.
Journal reference:
- Le, H., Bonthrone, A.F., Uus, A. et al. Autism common variants associated with white matter alterations at birth: cross-sectional fixel-based analyses of 221 European term-born neonates from the developing human connectome project. Transl Psychiatry (2025), DOI: 10.1038/s41398-025-03252-3, https://www.nature.com/articles/s41398-025-03252-3