Core laboratories must balance the efficiency of their NGS workflow with the quality of the data that they produce in providing numerous next generation sequencing (NGS) services relating to a wide scope of sample types, customers, and sequencing methods.
The expenses associated with library preparation begin to dominate the cost of the entire NGS workflow as the cost of sequencing falls.
A number of institutions are adopting the trend of reaction miniaturization in order to address this dominant expense in sequencing, effectively decreasing volumes to as low as 10% of the volume recommended by the manufacturer.
This article outlines a workflow for producing high-quality NGS libraries, even from low cellular input, via precise positive displacement microfluidic reagent dispensing.
The quality control data for quarter volume Illumina Nextera XT library preparation highlights the efficacy and applicability of reaction miniaturization with regards to single-cell sequencing.
Materials
- Alpaqua 384 Post Magnet Plate (PN A001222)
- Thermal Cycler
- Eppendorf twin.tec 384 plates (PN 0030129342)
- ThermoFisher Qubit 4 Fluorometer (PN Q33226)
- Illumina Nextera XT DNA Library Preparation Kit (PN FC-131-1096)
- FORMULATRIX® MANTIS® Liquid Handler (PN MANTV3.2)
- Illumina Nextera XT Index Kit V2 (FC-131-2001)
- Agilent 2100 Bioanalyzer (PN G2939BA)

Image Credit: FORMULATRIX
Methods
Quarter volume illumina Nextera XT library preparation
Eight murine neuronal cell cDNA samples (3 cell types; 1 ng DNA as input) were used in this pilot project to assess the quality of libraries produced at quarter volumes via the following protocol:
A. Tagment cDNA – 6.35 µL total reaction volume
- Start with 1.25 µL of input DNA in wells of a 384-well microplate
- Aspirate TD buffer into a 200 µL pipette tip* and place on LV chip on MANTIS. Dispense 2.5 µL of TD buffer into each sample containing well with MANTIS.
- Mix
- Aspirate ATM into a 200 µL pipette tip* and place on LV chip on MANTIS. Dispense 1.3 µL of ATM into each sample containing well with MANTIS.
- Mix
- Thermal Cycle:
- 55 °C for 5 min
- 10 °C Hold
- Aspirate NT Buffer into a 200 µL pipette tip* and place on LV chip on MANTIS. Dispense 1.3 µL of NT Buffer into each sample containing well with MANTIS.
- Mix
- Incubate at room temperature for 5 minutes
B. Amplify Libraries – 12.65 µL total reaction volume
- Aspirate NPM into a 200 µL pipette tip* and place on LV chip on MANTIS. Dispense 3.8 µL into each sample containing well with MANTIS.
- Manually pipette 1.25 µL of two index primers to samples so that each sample receives a unique combination of index primers.
- Mix.
- Thermal Cycle:
- 2 °C for 3 min
- 95 °C for 30 sec
- 12 cycles of 95 °C for 10 sec
- 55 °C for 30 sec
- 72 °C for 30 sec
- 72 °C for 5 min
- 10 °C Hold
C. Clean-up Libraries
- Aspirate AMpure XP beads into 200 µL pipette tip* and place on the HV chip on MANTIS. Dispense 10 µL into each sample containing well with MANTIS.
- Mix.
- Incubate at room temperature for 5 minutes
- Place plate on magnet and wait for 2 minutes
- Manually remove all supernatant (do not remove plate from magnet until step 11.).
- Add 50 µL of 80% EtOH to each sample containing well by utilizing continuous flow on MANTIS.
- Incubate for 30 seconds.
- Manually remove all supernatant while plate sites on magnet.
- Repeat steps 6-8.
- Air dry for 15 minutes.
- Remove plate from magnet.
- Aspirate RSB into a 200 µL pipette tip* and place on HV chip on MANTIS.
- Dispense 15 µL into each sample containing well with MANTIS.
- Mix.
- Incubate at room temperature for 2 minutes.
- Place plate on magnet.
- Incubate for 2 minutes.
- Manually transfer 12.5 µL from beads to a new plate.
*Aspiration volume = dispense volume per sample x number of samples + 10% safety factor. Use a 1000 µL pipette tip for aspiration volumes above 200 µL.
Results
Tracings from the Agilent BioAnalyzer were used to assess the quality of Nextera Libraries for each sample.
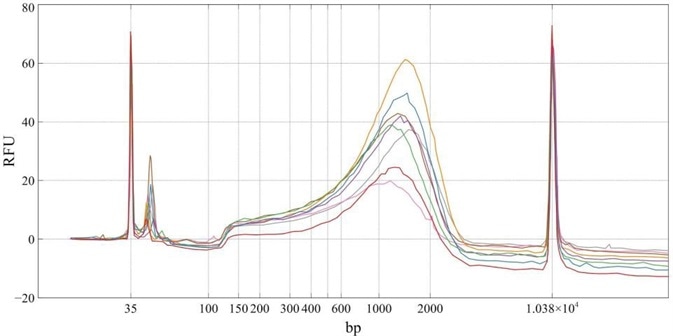
Figure 1. BioAnalyzer tracings of eight next generation sequencing libraries prepared from 8 unique samples of 1 ng murine neuronal cell cDNA. Image Credit: FORMULATRIX
Conclusion
The BioAnalyzer tracings showed that the composition of libraries prepared through quarter volume reaction miniaturization was acceptable and comparable to those prepared when following the full-volume protocol. Preparing libraries at quarter volumes leads to significant cost savings of 75% of the kit cost per reaction.
The MANTIS® Liquid Handler from FORMULATRIX® enables reaction miniaturization through precise microfluidic reagent dispensing at volumes as low as 100 nL and is easy to use, reliable, and consumable-free.
Acknowledgments
Produced from materials originally authored by Xi Chen from the Comprehensive Cancer Center at The Ohio State University and Thomas Rawlins from FORMULATRIX®.
About FORMULATRIX
FORMULATRIX® was established in 2002 to provide protein crystallization automation solutions. Since then, we've started developing other laboratory automation solutions including the next generation of liquid handlers using microfluidic technology.
Headquartered in Bedford, Massachusetts, we supply software and robotic automation solutions to leading pharmaceutical companies and academic research institutions around the world. Our team works tirelessly to provide the best products in the industry with support that is second to none.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.