One of the most common disinfectants utilized in both commercial and domestic and settings because of its favorable properties, including broad-spectrum antimicrobial activity and low cost, is sodium hypochlorite.1
It has also been included for use against COVID-19 on the environmental protection agency (EPA) list of disinfectants recommended. This has led to a fast rise in the production and utilization of sodium hypochlorite for disinfecting workplace and household environments.
While safe handling practices must be implemented together with the use of personal protective equipment (PPE), there is also a requirement for quality control to ensure manufacturers keep within the acceptable limits of sodium hypochlorite in industrial-strength and domestic and bleaches limit damage and exposure to humans.2
An iodometric titration is the standard technique used for establishing hypochlorite, which, although it is very useful, needs a number of chemical reagents and so can be costly and time consuming and when running numerous samples.
UV/Vis spectroscopy enables samples to be measured without the requirement for several different reagents and within just 30 seconds. The PerkinElmer LAMBDA® 365 UV/Vis spectrometer (Figure 1) gives a fast and simple solution for the measurement of sodium hypochlorite in a QA/QC setting where accuracy and speed are critical.

Figure 1. PerkinElmer LAMBDA 365 UV/Vis Spectrometer. Image Credit: PerkinElmer
Experimental conditions
Chemical reagents were bought from Merck™ and used as received. An iodometric titration is required to create the calibration curve in order to standardize the stock sodium hypochlorite solution.
In this instance, the solution was determined to contain 8.13% sodium hypochlorite. The calibration solutions were made, by volume to 50 mL, to cover a range of 0.001 – 0.05% NaOCl. Each solution measured by UV/Vis also included a 1 mL aliquot of 10% NaOH solution.
This was added to ensure the equilibrium between hypochlorous acid and hypochlorite, as shown, was determined on the side of hypochlorite, which is the species responsible for the instrument response.
HOCl ⇋ H+ + ClO–
Further to the calibration solutions, three independent validation solutions were also created, utilizing the same technique. The test solution was prepared by 200-fold dilution and addition of the 1 mL aliquot of NaOH solution and was a sample of commercial bleach.
All of the samples were quantified by utilizing the PerkinElmer LAMBDA 365 UV/Vis spectrometer with the instrumental parameters seen in Table 1. The measurements were performed using the absorbance at 292 nm.
Table 1. Instrumental parameters used for the measurement of sodium hypochlorite in disinfectants. Source: PerkinElmer
| Parameter |
Value |
| Range |
250 – 500 nm |
| Band Width |
1.0 nm |
| Data Interval |
1.0 nm |
| Scan Rate |
600 nm/min |
Calibration
By plotting the absorbance at 292 nm against concentration of sodium hypochlorite, the calibration curve could be created. Figure 2 shows the UV/Vis spectra of the calibration samples. Figure 3 shows the calibration curve and the R2 value was discovered to be 0.9999, which showed a high degree of linearity.
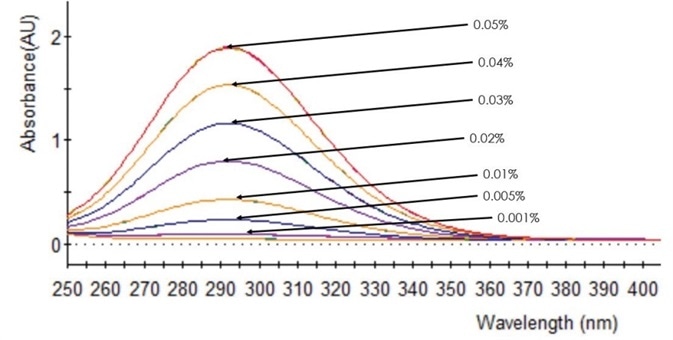
Figure 2. UV/Vis spectra of NaOCl calibration standards. Image Credit: PerkinElmer
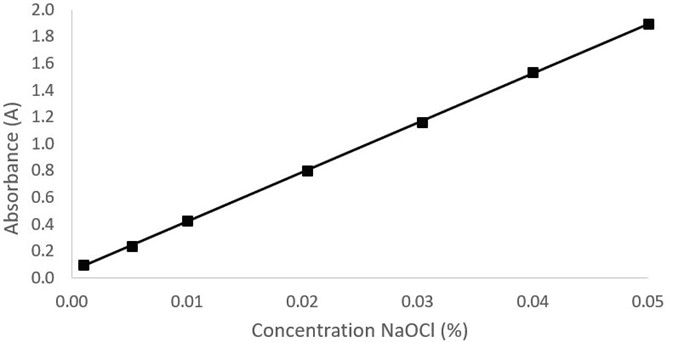
Figure 3. Plot of absorbance at 292 nm against concentration of NaOCl. Image Credit: PerkinElmer
Validation
Three independent validation samples were used to validate this method, as well as a sample of commercial bleach and Table 2 shows the results from this validation.
Table 2. Validation Results. Source: PerkinElmer
| Sample |
Calculated Concentration (%) |
| 0.003% |
0.0028 ± 0.00001 |
| 0.015% |
0.015 ± 0.00004 |
| 0.0420% |
0.043 ± 0.0001 |
| Bleach Sample |
0.027 ± 0.00005 |
In the bleach sample, the concentration of sodium hypochlorite was discovered to be 5.40% when corrected for dilution factor. In order to establish the precision of this technique, 10 replicates of the 0.015% validation sample were measured.
The relative standard deviation (RSD) was found to be 0.07%, showing a high level of precision. Five replicates of a blank solution were also measured to establish the limit of detection (LOD) of this technique using the equation:
LOD=yB+3sB
Subsequently, the limit of detection was discovered to be 0.0002% NaOCl, showing a high level of sensitivity in this technique.
Conclusion
UV/Vis spectroscopy supplies an accurate, fast technique for the measurement of sodium hypochlorite in disinfectants. This will enable analysts working in a QA/QC laboratory to save expenditure and time.
The PerkinElmer LAMBDA 365 provides a fast, high performance solution for those looking to measure sodium hypochlorite in disinfectants and can be utilized together with either UVExpress or UVWinLab software.
UVWinLab enables users to have maximum control over instrument parameters and provides advanced data analysis choices. Less experienced users may utilize UVExpress, which provides a simple point-and-click solution for both the data analysis and instrumental control.
References
- https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/chemical-disinfectants.html?CDC_AAref_Val=https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed 12/08/2020).
- https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19 (accessed 12/08/2020).
About PerkinElmer
As a global technology leader, PerkinElmer is taking action to harness the power of insights and transform them into knowledge to deliver innovative, differentiated solutions for our customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.