Key hygiene supplies are now in high demand due to the COVID-19 outbreak, to such an extent that there are now critical supply shortfalls. Alcohol-based hand sanitizer is one of the most important of these supplies.
The food and drug administration (FDA) has created a guidance document for the compounding of certain alcohol-based hand sanitizer products during this pandemic in order to cope with this shortfall.1 There are two formulations that have been approved for compounding by facilities with the ability to produce hand sanitizer.
They are based on formulations that are recommended by the World Health Organisation (WHO):2
- Hydrogen Peroxide (0.125% v/v)
- Ethyl alcohol (80% v/v) OR Isopropyl alcohol (75% v/v)
- Sterile or distilled water (Remainder of volume)
- Glycerol (1.45% v/v)
The content and type of alcohol are the key parameters that must be considered in compounding. It has been determined that the aforementioned concentrations utilized in such formulations are the most effective. It has also been established that hand sanitizer with an alcohol concentration that is less than 60% (v/v) could leave the user at higher risk of infection as it is not effective.3
Considering exposure to the body, it should be noted that the alcohol type should also be safe for humans. Methanol/Wood Alcohol is harmful and should not be used, while Ethanol or Isopropyl Alcohol (IPA) are okay to use.
There have been instances of hand sanitizers that have partial or complete methanol content, and that would be harmful to users. Prior to verifying the composition, the PerkinElmer Hand Sanitizer Analyzer is also able to detect the presence of methanol or other threats.
Experimental
In order to prepare validation and calibration standards, stock solutions of ethanol and isopropanol (Sigma-Aldrich) were utilized. Calibration standards from 0-90% (v/v) ethanol and isopropanol were prepared by volume.
Each standard was made up so that they all possessed the same quantity of glycerol and hydrogen peroxide (1.45 and 0.125% v/v respectively) and the correct proportion of the alcohol. Deionized water was used to make solutions up to 50 mL.
All of the samples were quantified using the PerkinElmer Spectrum Two+ FT-IR spectrometer, as seen in Figure 1, with attenuated total reflectance accessory using the parameters exhibited in Table 1.
Table 1. Parameters used for the measurement of hand sanitizer samples. Source: PerkinElmer
| Parameter |
Value |
| Range |
4000 – 550 cm-1 |
| Resolution |
4 cm-1 |
| Number of Scans |
4 |
| Corrections |
Atmospheric Compensation |
Figure 1. PerkinElmer Spectrum Two+ FT-IR spectrometer with ATR accessory. Image Credit: PerkinElmer
Results and discussion
In order to produce starter calibrations for the two different hand sanitizer models, the Beer-Lambert law was utilized. The ethanol-based hand sanitizer model was produced based on the standard curve derived from the area of a peak at 1045 cm-1, which corresponds to the C-O stretch in a primary alcohol.
Similarly, the isopropanol-based hand sanitizer model was produced based on the area of a peak at 1131 cm-1, which corresponds to the C-O stretch in a secondary alcohol. For example, Figure 2 shows FT-IR spectra containing 80% of the active alcohol ingredient in both types of hand sanitizer.
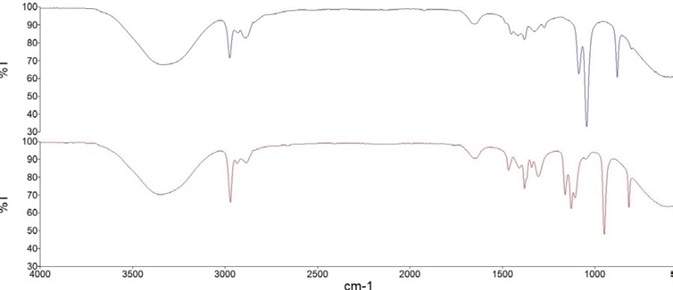
Figure 2. FT-IR spectra of hand sanitizer calibration solutions containing 80% v/v Ethanol (blue) and 80% v/v Isopropanol (red). Image Credit: PerkinElmer
The calibration curves produced by utilizing the peaks previously discussed for both ethanol and isopropanol can be seen in Figure 3, and they have been fit to a linear regression curve. The calibration models may be performed in one of three possible ways using PerkinElmer’s Spectrum Two+ FT-IR hand sanitizer analyzer:
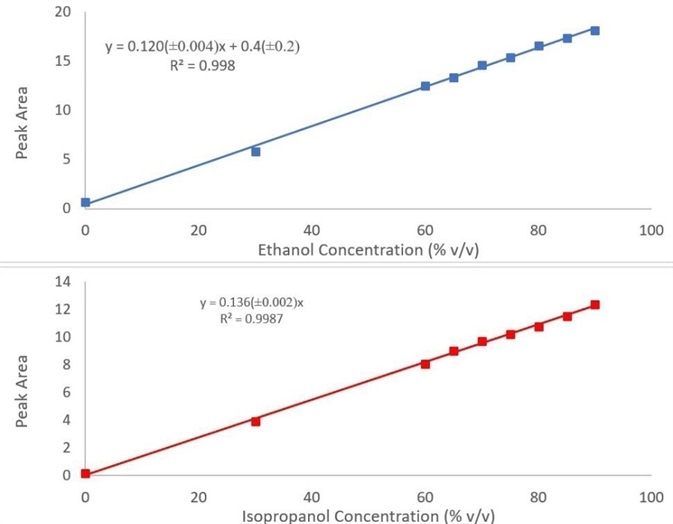
Figure 3. Calibration curves for ethanol (top) and isopropanol (bottom) in hand sanitizer. Image Credit: PerkinElmer
- Implementing a Touch method
- Utilizing the Quant function in Spectrum 10™
- Utilizing the Predict function in Spectrum Quant™
Two samples were utilized to validate each model to show the prediction accuracy of the starter calibrations. This step consisted of testing known concentrations of each sample type against the standard curve. Table 2 shows the results of the validation.
Table 2. Validation results for ethanol and isopropanol-based hand sanitizer models. Source: PerkinElmer
| Model |
Concentration (%) |
Predicted Concentration (%) |
| Ethanol |
43 |
41% |
| 73 |
73% |
| Isopropanol |
43 |
41% |
| 73 |
72% |
Detection of methanol in hand sanitizer
An increasingly important issue during the COVID-19 outbreak has been the discovery of methanol in some hand sanitizer. The FDA issued warnings in the month from July to August 2020 due to methanol contamination against using 130 different brands of hand sanitizer.
Together with PerkinElmer's Adulterant ScreenTM algorithm, IR spectroscopy can be utilized as a screening tool for the detection of methanol in hand sanitizer. It can detect methanol contamination down to 300 ppm (0.03%), far exceeding the limits needed to adhere to FDA regulation (630 ppm).
Spectrum Touch enables this analytical step to be included in a one-click solution for establishing both presences of methanol and alcohol concentration. Figure 4 shows an example of the results page from a sample that was discovered to contain methanol.
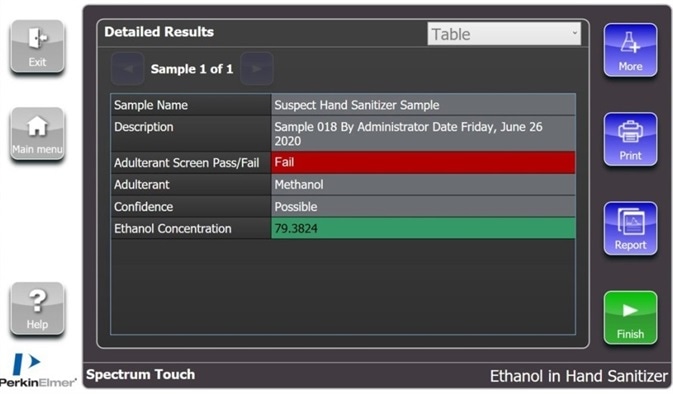
Figure 4. Example results page from a sample containing approximately 80% ethanol which has been contaminated with methanol. Image Credit: PerkinElmer
Conclusion
The PerkinElmer Spectrum Two+ FT-IR spectrometer supplies a reliable, fast technique to quantify the ethanol and isopropanol content of hand sanitizers compounded according to WHO and FDA-approved formulations.
The quantitative models included show strong correlation with R2 values of 0.998 and 0.999 for ethanol and isopropanol concentration, respectively. The optional adulterant screen enables users to detect methanol down to 300 ppm, ensuring sample safety and easily fulfilling FDA requirements.
The complete analyzer includes a starter calibration for hand sanitizer ethanol and isopropanol content and Spectrum Touch software, which enables the user to work with workflow orientated techniques.
It is also possible to add the methanol adulterant screen to the touch macro to supply a simple, quick method to screen for this potential contaminant. This enables analysts to gather vital information about sample formulations quickly and at the compounding site.
References
- https://www.fda.gov/ (Accessed 19/03/2020)
- WHO Guidelines on Hand Hygiene in Health Care, Part I, Chapter 12, Page 49
- WHO Guidelines on Hand Hygiene in Health Care, Part I, Chapter 10, Page 28
About PerkinElmer
As a global technology leader, PerkinElmer is taking action to harness the power of insights and transform them into knowledge to deliver innovative, differentiated solutions for our customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.