Ethanol, also known as ethyl alcohol, is one of the most commonly used volume organic chemicals utilized in the manufacture of consumer products, including personal care products and household cleaners.
It is also an essential chemical when used in industrial products such as printing inks and adhesives, as well as sanitizers which have become central to the daily sanitation needs of humans around the world.
It is crucial that the quality of ethanol used in these products is carefully monitored to protect human and animal health. This article presents an analysis of trace level impurities that could be found in ethanol as per the United States Pharmacopeia (USP) guideline.
Firstly, the analysis of USP grade Ethanol is described in accordance with USP recommended conditions and procedures. The second section details a procedure that has been modified to generate excellent results with a significant improvement in data throughput and a considerable reduction in laboratory sample preparation time.
Instrumentation
The PerkinElmer Clarus® 690 GC with built-in autosampler was used to carry out these experiments. Configuration of the Clarus 690 GC was in arrangement with a capillary injector and dual flame ionization detector (FID), which possesses an extensive range.
Installation of a PerkinElmer Elite 624 and BAC-1 columns was positioned in the injector via a two-hole ferrule or a “Y” splitter as displayed in Figure 1. The GC conditions necessary for the analysis are detailed in Table 1.
Figure 1. 30 m 0.32 mm Elite 624 and Elite-BAC columns used in this study. Image Credit: PerkinElmer
Experimental
The GC conditions can be seen in Table 1.
Chromatography conditions
Table 1. Chromatography conditions. Source: PerkinElmer
| GC Parameters |
| Instrument |
PerkinElmer Clarus 690 GC |
| Carrier Gas |
Helium |
| Columns |
Elite-624 30 m 0.32 mm 1.8 μm N9316203
Elite-BAC1 30 m 0.32 mm 1.8 μm N9315071 |
| Column Pneumatics |
Flow: 1 mL/minute Split: 50 mL/min |
| Autosampler Parameters |
| Syringe Size |
5 μL |
| Injection Volume |
1 μL |
| Injection Speed |
Normal |
| # of Plunges |
8 times |
| Pre-washes |
4 |
| Sample Washes |
2 |
| Post Washes |
2 |
| Viscosity |
5 |
| Injector Parameters |
| Injector |
Type: S/S Temp: 240 °C |
| Detector Parameters |
| Type |
FID |
| Temperature |
300 °C |
| Range |
1 |
| Att |
-6 |
| Hydrogen |
30 mL/min |
| Air |
450 mL/min |
| Data Rate |
12.5 pt/sec |
| Oven Parameters |
| Oven Initial Temperature |
40 °C |
| Oven Initial Hold |
5 minutes |
| Ramp Rate |
30 °C/minute |
| Final Temperature |
240 °C |
| Final Time |
4 minutes |
| Oven Maximum |
260 °C |
| Equilibration Time |
0 |
Data acquisition
Data acquisition and data processing were conducted with the TotalChrom™ chromatography data system (CDS) software.
Sample preparation
An aliquot of the Ethanol sample was introduced to a 2 mL autosampler vial and arranged in place in the integrated autosampler of the Clarus 690 Gas Chromatograph (GC).
Standard preparation:
Table 2. Preparation of standards. Source: PerkinElmer
| Impurity |
Std Conc. |
Desired Conc.PPM |
Final Vol. |
Amt. Used μL |
| Acetone |
999000 |
4400 |
25 |
110 |
| 1-propanol |
999000 |
1000 |
25 |
25 |
| Ethyl Acetate |
999000 |
2200 |
25 |
55 |
| 2-butanol |
999000 |
6200 |
25 |
155 |
| IsoButanol |
999000 |
21700 |
25 |
543 |
| 1- butanol |
999000 |
1000 |
25 |
25 |
| 1-methyl-1- butanol |
999000 |
4100 |
25 |
102.6 |
| Amyl Alcohol |
999000 |
4100 |
25 |
102.6 |
10 mL of ethanol was used to dilute 0.3 mL of the above standard for running in the GC system.
Results and discussion
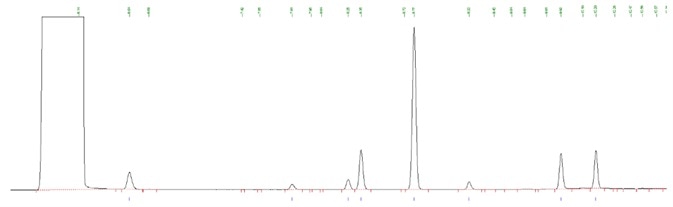
Figure 2. Impurity standard using 624 column. Image Credit: PerkinElmer
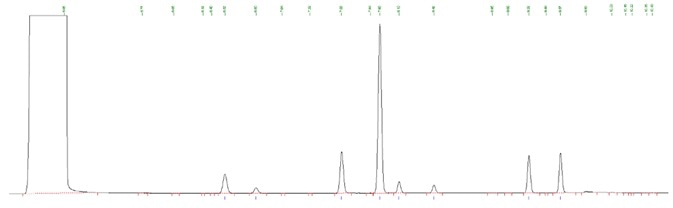
Figure 3. Impurity standard using BAC column. Image Credit: PerkinElmer
Table 3. Impurities %RSD of Ethanol and IPA standards. Source: PerkinElmer
| Ethanol |
G43
Column |
Confirmatory
Column |
Isopropanol |
G43
Column |
| Compound |
Precision
% RS |
Precision
% RSD |
Compound |
Precision
% RSD |
| Acetaldehyde |
0.78 |
1.18 |
Ethyl Ether |
1.95 |
| Methanol |
1.17 |
1.25 |
Acetone |
1.69 |
| Benzene |
1.32 |
1.07 |
Diisopropyl |
1.42 |
| Acetal |
0.97 |
1.03 |
1-Propanol |
1.33 |
| 4-Methyl-2-Pentanol |
0.92 |
0.96 |
2-Butanol |
1.31 |
Summary
It has been shown that utilizing both a G43 (624) and BAC-1 as the confirmation column demonstrates an excellent %RSD of impurities found in hand sanitizers. A rapid 10 minute analytical run time facilitates maximum laboratory productivity.
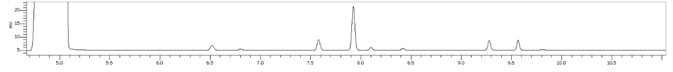
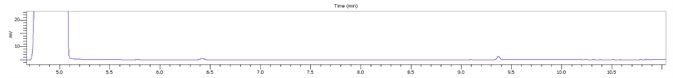
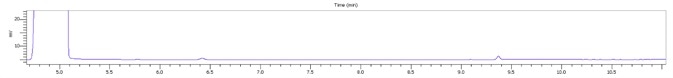
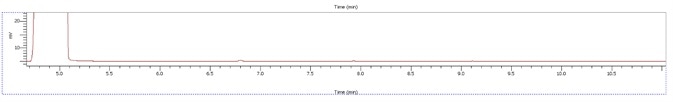
Figure 4. Stacked plot of standard and three locally purchased hand sanitizers. Image Credit: PerkinElmer
About PerkinElmer
As a global technology leader, PerkinElmer is taking action to harness the power of insights and transform them into knowledge to deliver innovative, differentiated solutions for our customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.