Isopropyl alcohol, also known as Isopropanol or IPA, is typically used as a sanitizer specifically for use on the surface of the skin prior to an injection of a vaccine or medicine. This solution must not contain impurities that are known to be a health hazard, thus, the necessity for quality testing.
The USP has issued specifications for the maximum levels of known IPA targeted impurities, including ethyl ether, acetone, diisopropyl ether, 1-propanol, and 2-butanol at 0.1% or less. This is described and analyzed in two parts; the first, the USP recommends conditions generating a 42 minute analysis time. The second section details a rapid method for accurate and precise results in under 6 minutes.
Instrumentation
The PerkinElmer Clarus® 690 GC with integrated autosampler was used to carry out these experiments. The Clarus 690 GC was configured with a capillary injector and dual wide-range flame ionization detector (FID). Installation of a PerkinElmer Elite 624 and BAC-1 columns was positioned in the injector via a two-hole ferrule or a “Y” splitter as displayed in Figure 1. The GC conditions necessary for the analysis are detailed in Table 1.
Experimental
Sample preparation
An aliquot of the IPA sample was dispensed into a 2 mL autosampler vial and placed in the built-in autosampler of the Clarus 690 Gas Chromatograph (GC).
Standard preparation
The calibration of Isopropanol method is the common weight percent method and uses the Not Less Than (NLT) technique.
Table 1. Chromatography conditions. Source: PerkinElmer
| GC Parameters |
|
| Instrument |
PerkinElmer Clarus 690 GC |
| Carrier Gas |
Helium |
| Columns |
Elite-624 30 m 0.32 mm 1.8 μm N9316203
Elite-BAC1 30 m 0.32 mm 1.8 μm N9315071 |
| Column Pneumatics |
LCV: 35 cm/sec Split flow: 20 mL/min |
| Autosampler Parameters |
|
| Syringe size |
5 μL |
| Injection volume |
1 μL |
| Injection speed |
Normal |
| # of plunges |
8 |
| Pre-washes |
0 |
| Sample washes |
2 |
| Post washes |
4 |
| Viscosity delay |
0 |
| Injector Parameters |
|
| Injector |
Type: CAP Temp: 200 °C |
| Detector Parameters |
|
| Type |
WR-FID |
| Temperature |
230° C |
| Range |
20 |
| Att |
0 |
| Hydrogen |
30 mL/min |
| Air |
450 mL/min |
| Data rate |
12.5 pt/sec |
| Oven Parameters |
|
| Oven initial temperature |
40° C |
| Oven initial hold |
12 minutes |
| Ramp Rate |
10 °C/minute |
| Final Temperature |
240 °C |
| Final Time |
10 minutes |
| Oven maximum |
260 °C |
| Equilibration time |
0 |
The GC conditions, in accordance with the USP protocol, are displayed in Table 1. PerkinElmer developed a rapid method as an alternative using the same columns as described later in this article.
Result = (RU/RT) x 100
RU = Peak response of isopropyl alcohol
RT = Sum of all the peak responses
Acceptance criteria: NLT 99.0%
Data acquisition
Data acquisition and data processing were conducted by employing the TotalChrom™ chromatography data system (CDS) software.
Calibration
The analytes that will be targeted for impurities in IPA are ethyl ether, acetone, diisopropyl ether, 1-propanol and 2-butanol at 0.1% each in IPA. (RS system suitability solution)
Result = (RU/RT) x 100
RU = Peak response of each individual impurity in the Sample Solution
RT = Sum of all the peak responses in the Sample Solution
Suitability requirements
Resolution: NLT 1.5 between acetone and isopropyl alcohol
Relative standard deviation (% RSD): NMT 2.0% for the isopropyl alcohol peak
Tailing factor: NMT 2.0 for the isopropyl alcohol peak
Signal-to-noise ratio: NLT 10 for any of the following peaks: ethyl ether, acetone, diisopropyl ether, 1-propanol, and 2-butanol
IPA RS impurities standards. Image Credit: PerkinElmer
Results and discussion
Due to the fact numerous organic compounds are present, and a number of which co-elute, dual column confirmation was employed to help impede any reporting of false negatives. This technique is also used by the forensic industry for blood alcohol investigation.
The USP method applies a G43 (common name 624) phase as the reporting column for results and takes into account a column of different polarity stationary phase for the confirmatory column.
The BAC 1 phase was selected for its chromatographs and capacity to separate these compounds effectively. Additionally, the forensics industry has thoroughly investigated this technique as the reporting column for blood alcohol investigation. The IPA sample and RS System suitability sample are displayed below in Figures 1 and 2.
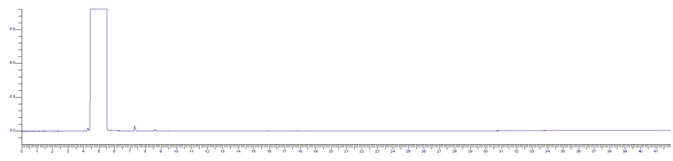
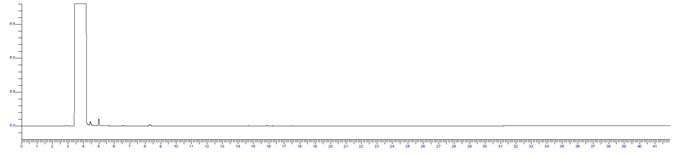
Figure 1. HPLC grade IPA sample. Image Credit: PerkinElmer
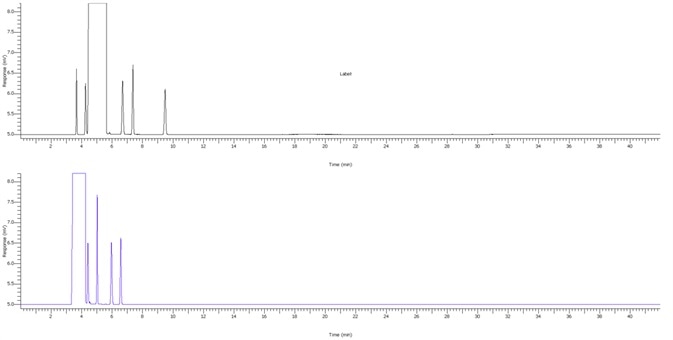
Figure 2. IPA RS System suitability standard at 0.1%. Image Credit: PerkinElmer
Sample results
An IPA sample of unknown origin was analyzed to determine if it meets USP criteria of all targets must be less than 0.1%. The sample passed with ease and the results/calculations are shown in Table 2. To determine repeatability, a set of 8 replicates were run, as illustrated in Table 3.
Table 2. Calculation of impurities in IPA. Source: PerkinElmer
| Target |
Area |
Area % |
Spec |
| Ethyl Ether |
0 |
|
0.10% |
| Acetone |
293 |
0.007499 |
0.10% |
| Diisopropyl |
0 |
|
0.10% |
| 1-Propanol |
390 |
0.009982 |
0.10% |
| 2-Butanol |
0 |
|
0.10% |
| Total area of all peaks |
3906982 |
|
|
Table 3. 8 replicates of IPA results. Source: PerkinElmer
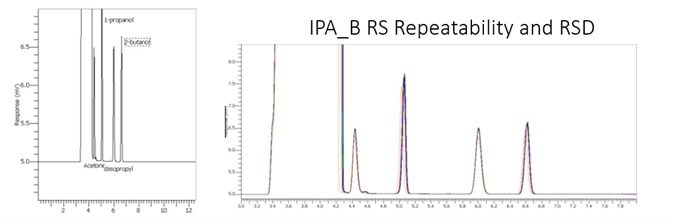
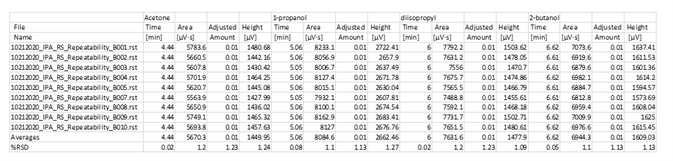
Table 4. Precision of Impurities in IPA. Source: PerkinElmer
| Isopropanol |
|
|
| Compound |
Action Limit (μL/L) |
Precision% RSD |
| Ethyl Ether |
1000 |
1.0 |
| Acetone |
1000 |
1.7 |
| Diisopropyl |
1000 |
1.0 |
| 1-Propanol |
1000 |
1.0 |
| 2-Butanol |
1000 |
1.0 |
Summary part one
It was established that the PerkinElmer dual-column FID Clarus 690 GC could effectively analyze impurities in IPA employing the USP method with outstanding accuracy and selectivity for alcohols used in the manufacture of hand sanitizer by USP methodology
Alternate method
Alternate introduction
While the analysis of isopropanol by the USP method as previously detailed works well with good results. There are some items that could be refined to boost lab productivity. Within the first 10 minutes of a 42 minute run time all the target compounds elute in the analysis of isopropanol.
The temperature ramp’s function at the end of the run is to clean and simplify the column for the next run. If the oven ramp rate temperature is raised from 10 to 50C/min this will reduce the total analysis time significantly.
Additionally, the oven ramp could be initiated immediately after the elution of isopropanol as significant resolution of the remaining target analytes remains. Reduction of this analysis can be lowered as much as 7 times.
Alternate experimental
The Instrument configuration is identical to that as described above.
Sample/standard preparation
Sample and Standard preparation was the same as those described in the procedure above.
Chromatography conditions
While similar to the conditions displayed in Table 1, the chromatography column and condition have been modified for increased speed and/or improved data quality. The modified parameters in contrast to those in Table 1 are shown below in Table 5.
Table 5. Alternate instrument parameters for impurities in Isopropanol. Source: PerkinElmer
| GC Parameters |
|
| Instrument |
PerkinElmer Clarus 690 GC |
| Carrier Gas |
Helium |
| Columns |
Elite-624 30 m 0.32 mm 1.8 μm N9316203
Elite-BAC1 30 m 0.32 mm 1.8 μm N9315071 |
| Column Pneumatics |
LCV: 40 cm/sec Split flow: 20 mL/min |
| Autosampler Parameters |
|
| Syringe size |
5 μL |
| Injection volume |
1 μL |
| Injection speed |
Normal |
| # of plunges |
6 |
| Pre-washes |
2 |
| Sample washes |
2 |
| Post washes |
2 |
| Viscosity delay |
0 |
| Injector Parameters |
|
| Injector |
Type: CAP Temp: 200 °C |
| Detector Parameters |
|
| Type |
WR-FID |
| Temperature |
230 °C |
| Range |
20 |
| Att |
-6 |
| Hydrogen |
30 mL/min |
| Air |
450 mL/min |
| Data rate |
12.5 pt/sec |
| Oven Parameters |
|
| Oven initial temperature |
40 °C |
| Oven initial hold |
5 minutes |
| Ramp Rate |
70 °C/minute |
| Final Temperature |
230 °C |
| Final Time |
1 minute |
| Oven maximum |
260 °C |
| Equilibration time |
0 |
Results and discussion
The overall w/result and discussion of the alternate method are almost comparable to the conventional method shown above. Most notable is the significant reduction in analysis time, thus facilitating greater laboratory sample throughput.
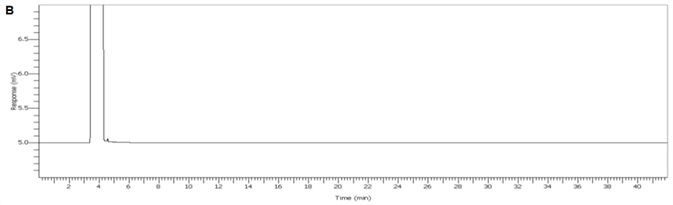
Figure 3. Chromogram of IPA blank. Image Credit: PerkinElmer
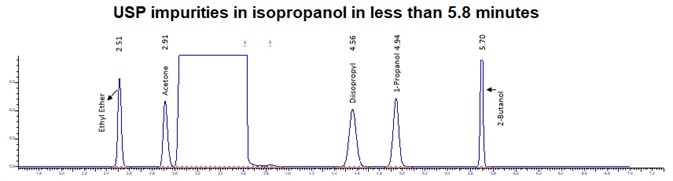
Figure 4. IPA impurities in less than 5.8 minutes using alternate analysis method. Image Credit: PerkinElmer
Figure 4 displays a clean IPA blank of both FID channels with interfering sample carryover for enhanced confidence of the reported results. Figure 5 demonstrates exceptional separation of the targeted IPA impurities in under 5.8 minutes to facilitate a seven-fold decrease in the analysis run time.
Table 6. Fast analysis method performance using column A. Source: PerkinElmer
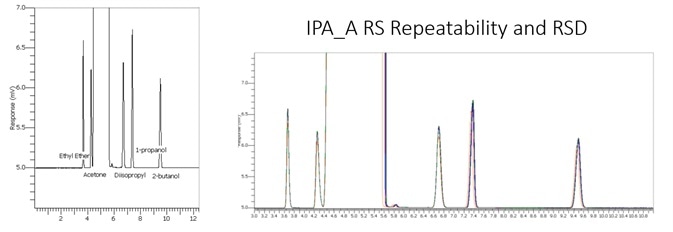
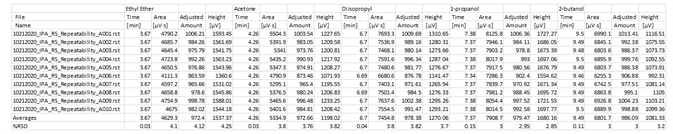
Table 7. Fast analysis method performance using column B. Source: PerkinElmer
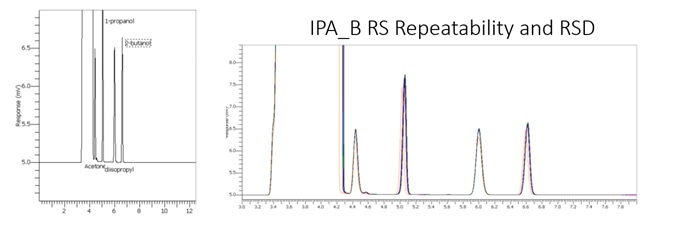
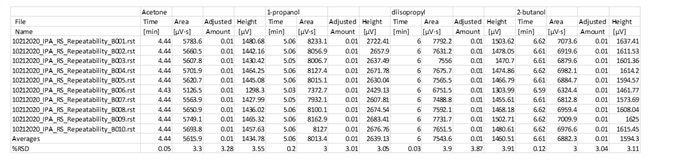
Table 8. Alternate results for Precision of Impurities in IPA. Source: PerkinElmer
| Isopropanol |
|
| Compound |
Precision% RSD |
| Ethyl Ether |
1.95 |
| Acetone |
1.69 |
| Diisopropyl |
1.42 |
| 1-Propanol |
1.33 |
| 2-Butanol |
1.31 |
Table 6 and 7 displayed outstanding performance of this rapid alternate analysis method on both instrument channels. The class and excellence of the repeatability is outlined in Table 8.
Summary part two
The modified oven parameters facilitated faster run times, reducing them from 42 minutes right down to 5.8 minutes with superb accuracy and precision. The GC system can run both the official extended method or the enhanced throughput method with a simple software method selection offering optimal performance based on the current needs of the laboratory.
Reference
About PerkinElmer
As a global technology leader, PerkinElmer is taking action to harness the power of insights and transform them into knowledge to deliver innovative, differentiated solutions for our customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.