An end-to-end solution is a completely functional solution delivering a self-contained service, system, or process stage from start to finish. Individual components are typically sourced and managed through a single supplier within a fully integrated solution.

Image Credit: Pall Corporation
By focusing supply on a single point of ownership, this type of solution has the benefit of decreasing supply chain risk. It is for this reason that a fully integrated process can become a trusted partnership between supplier and drug manufacturer.
As part of an end-to-end solution, integration is about bridging gaps and connecting processes efficiently whilst considering the full life cycle of any pharmaceutical product. When freezing drug substances, process disconnection often happens just before freezing when downstream processing is complete.
It can also be seen after freezing when frozen drug substances are transported for final fill and finish stages at contract manufacturing organizations (CMOs). More innovative integrated solutions would be especially beneficial.
They are able to strengthen process stages which have the potential to be disconnected whilst sharing value to both manufacturer and supplier via better planning and process alignment.
What does an integrated solution look like?
The aim of an integrated solution is to achieve a seamless connection between interlinked process steps. This can be attained when individual processes are viewed as a linear pathway that can be streamlined to permit an increase in time, efficiency, and product yield.
The steps immediately upstream and downstream of this operation must be carefully considered and linked in the context of freezing bulk drug substances.
When considering freeze-thaw, an integrated end-to-end solution begins where the drug substance is filled into the preferred containers, frozen, transported and culminates in thawing of drug substance for formulation and filling.
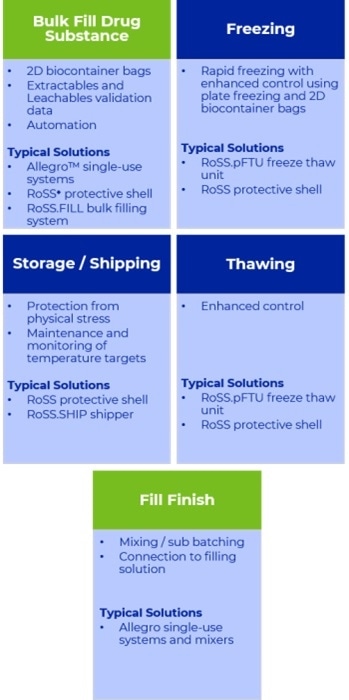
An Integrated Approach to Freeze / Thaw Solutions. Image Credit: Pall Corporation
Processes from filling to freezing can then be bridged through the use of 2D biocontainer bags and are driven by fast, controlled plate freezing and the advantages it offers through consistent homogeneity, quality and drug substance activity.
The choice of single-use technology helps define the choices for the automation of filling to streamline the process and control risk. This also supplies an opportunity to build in design elements to facilitate disconnection from the filling process.
Enhanced process connectivity is advantageous for the validation of an integrated solution. Data can be repurposed for all materials with a common contact surface, specifically that referring to the extractables and leachables assessment which forms a key part of the safety profile assessment of the final drug product.
Specifying product contact surfaces like those in filling or mixing systems minimizes the validation burden and decreases the potential set of leached materials in the final drug product.
With the desired freezing process in mind, it is beneficial to choose biocontainer bags that possess a low extractables profile and validation data at the target temperature, usually as low as -80°C.
Complete assurance of robustness is provided by comprehensive transport validation reports and data which comply fully with ASTM International protocol and International Safe Transit Association (ISTA) guidelines (3E and 3H).
This validation supports protective shells which are able to maintain the geometry of the frozen biocontainer bags during transit to CMOs.
After this, the controlled thawing of the bulk drug substance with the optimized design of all consumables permits a simple, safe transfer of thawed bulk to the fill-finish operations with the appropriate disconnection and connection technology, sharing the validation data for any common components which were utilized during the bulk filling.
A typical process may flow seamlessly with shared validation data and system design principles supporting high performance and robust, streamlined operations by utilizing these points of integration.
About Pall Corporation

Pall Corporation is a global leader in high-tech filtration, separation, and purification, serving the diverse needs of customers across the broad spectrum of life sciences and industry.
Pall Life Sciences provides cutting-edge solutions for customers who discover, develop and produce biotech drugs, vaccines, cell therapies and classic pharmaceuticals.
Our advanced medical technologies are often a patient’s last line of defense from dangerous pathogens. And our food and beverage products provide critical protection from contaminants during various manufacturing steps.
Pall Industrial serves customers in the microelectronics, aerospace, fuels, petrochemical, chemical, automotive, and power generation industries.
Our products play key roles in:
- Manufacturing innovative semiconductors and consumer electronics
- Filtration for commercial and military aerospace vehicles
- Maintaining reliability of essential industrial equipment
- Addressing mounting water quality, scarcity and demand issues
- Helping energy companies maximize production and develop commercially successful next generation fuels
Headquartered in Port Washington, New York, Pall has offices and plants throughout the world.
For more on how we enable a greener, safer future, visit Pall - Enabling A Greener, Safer Future.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.