Sponsored Content by BGI GenomicsReviewed by Olivia FrostMay 24 2023
Homologous Recombination Deficiency (HRD) is a biomarker that enables the prediction of breast cancer treatment with first-line platinum-based chemotherapy or ovarian cancer treatment with PARP inhibitors.
However, there is a lack of published research regarding platinum-based treatment prediction with HRD as a biomarker in ovarian cancer patients, particularly in the Chinese population.
The first China prospective cohort study was performed by BGI Genomics’ clinical researcher Dr. Shao Di in combination with the Fudan University Shanghai Cancer Center team.
This study was published in the Journal of Ovarian Research, and the findings reveal that HRD testing can accurately predict the sensitivity of platinum-based chemotherapy for patients with ovarian cancer.

The study results were published in the Journal of Ovarian Research. Image Credit: BGI Genomics
Method
The study involved 240 patients with primary treatment of high-grade serous ovarian cancer (HGSOC) admitted to the Cancer Hospital of Fudan University between January 2016 and September 2018.
Patients received more than two cycles of platinum-containing adjuvant chemotherapy after surgery and were classified as platinum-sensitive (Pt) and platinum-resistant according to the time of ovarian cancer recurrence.
All patients underwent a gene panel test screening for 68 HRR genes to evaluate the mutation status of HRR-related genes, e.g., BRCA. Of these, 118 samples were HRD tested to evaluate the telomere allele disequilibrium (TAI), loss of heterozygosity (LOH), and large segmental recombination abnormality (LST) status.
The Kaplan-Meier method was utilized to plot the survival curve and the efficacy of these tests in patient outcome prediction.
Results
Of the 240 HGSOC patients included in the study, 82.5% had Pt-sensitive cancer with a platinum-free interval greater than six months. The study demonstrates that 31.2% of patients had BRCA gene mutations, 53 in BRCA1 and 22 in BRCA2, as shown in Figure 1. In addition, 25% of patients had mutations in non-BRCA/HRR genes.
The percentage of patients with positive HRD status was 64.4%. These figures indicate that a greater proportion of Chinese ovarian cancer patients benefit from PARP inhibitor maintenance therapy in comparison to Western populations.
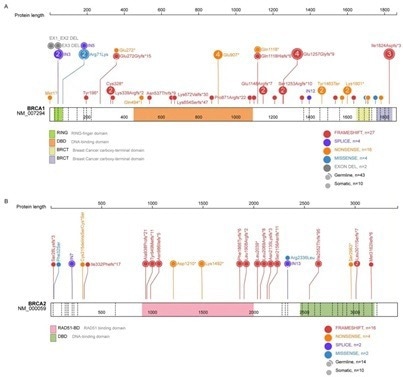
Figure 1. Distribution of BRCA1 and BRCA2 gene mutations. 31.2% of patients had BRCA gene mutations, 53 in BRCA1 and 22 in BRCA2. Image Credit: BGI Genomics
HRR gene mutation and HRD status are strongly correlated with platinum chemotherapy sensitivity in patients with HGSOC.
The results demonstrated that the HRD score of platinum treatment-sensitive patients was slightly higher than that of Pt-resistant patients (Figure 2A).
Further analysis revealed that the rate of Pt-sensitive patients in the HRD+ BRCAwt group and HRD+ BRCAm group was substantially greater than in the HRD-BRCAwt group, as shown in Figure 2B.
The study also discovered that Pt-sensitive patients were more enriched in the BRCA mutation group and non-BRCA HRR mutation group than in the HRR wt group.
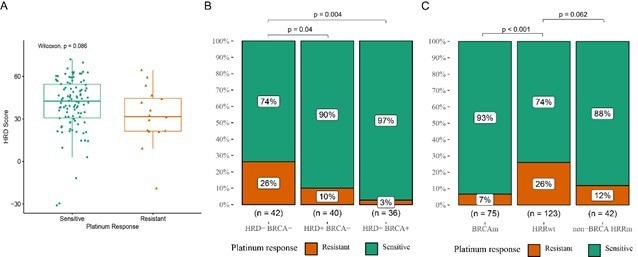
Figure 2. Correlation between HRD score, HRR mutation, HRD status and response to platinum chemotherapy. Image Credit: BGI Genomics
Analysis demonstrated that patients with positive HRD status had a significantly longer progression-free survival (PFS) compared to those with negative HRD status (median PFS: 30.5 months vs. 16.8 months, p=0.001 - Fig.3A)
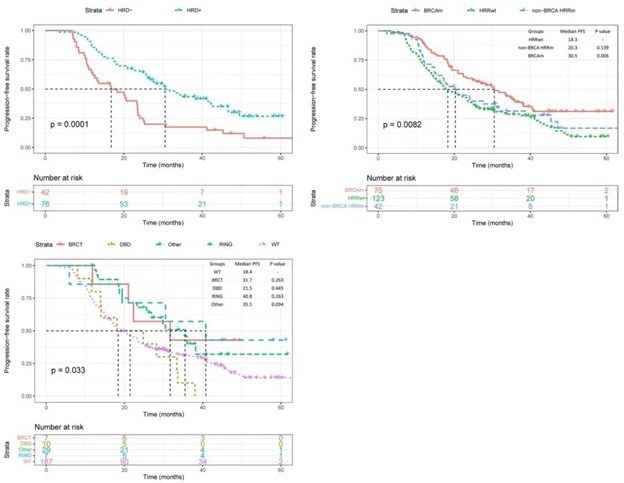
Figure 3. KM Survival Analysis Curves Grouped by HRD Status, HRR Gene Mutation, and BRCA1 Mutation Domain. Image Credit: BGI Genomics
Conclusion
This study revealed for the first time that HRD is a valid biomarker tool for the prediction of sensitivity to platinum-based chemotherapy in a prospective cohort of unselected Chinese ovarian cancer patients.
The study also discovered that there is a higher rate of HRD positivity in Chinese ovarian cancer patients compared to Western populations, indicating that HRD testing has the potential to screen a greater proportion of the Chinese PARP inhibitor population to enable precision therapy.
About BGI Genomics
BGI Genomics is the world's leading integrated solutions provider of precision medicine, now serving customers in more than 100 countries.
They provide academic institutions, pharmaceutical companies, healthcare providers, and other organizations with integrated genomic sequencing, proteomic services, clinical testing, and solutions across a broad range of applications.
They have more than 20 years of genomics experience helping customers and partners achieve their goals by delivering rapid, high-quality results using a broad array of cost-effective, cutting-edge technologies, including their own innovative DNBSEQ™ sequencing technology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.