Sponsored Content by BGI GenomicsReviewed by Olivia FrostSep 6 2023
Preeclampsia is a serious pregnancy disorder characterized by the presence of proteins in urine and high blood pressure. The disorder affects 2-4% of pregnant women globally, causing approximately 46,000 maternal deaths and 500,000 fetal and newborn deaths annually.
Predicting risk and developing treatments for preeclampsia is challenging due to the complexity and heterogeneity of the disorder.
Genomics Clinical Research Senior Manager Dr. Zhou Si and a team of scientists recently conducted a study that was published in the American Journal of Obstetrics and Gynecology. This study has shed light on a potential diagnostic technique for preeclampsia using plasma cell-free RNA (cfRNA).
Method and findings
CfRNA is released into the bloodstream by cells and may be utilized to investigate various diseases, including preeclampsia. cfRNA comprises different RNA biotypes such as microRNA, messenger RNA, and long noncoding RNA.
In the study described above, the research team conducted an analysis of cfRNA from 917 pregnancies, comprised of 202 pregnancies affected by preeclampsia before the onset of symptoms and 715 healthy pregnancies.
The key findings of this study include the following:
1. A total of 77 genes were detected, including microRNA (26%) and messenger RNA (44%), all of which were expressed differentially in mothers with preterm preeclampsia before symptom onset and in healthy mothers.
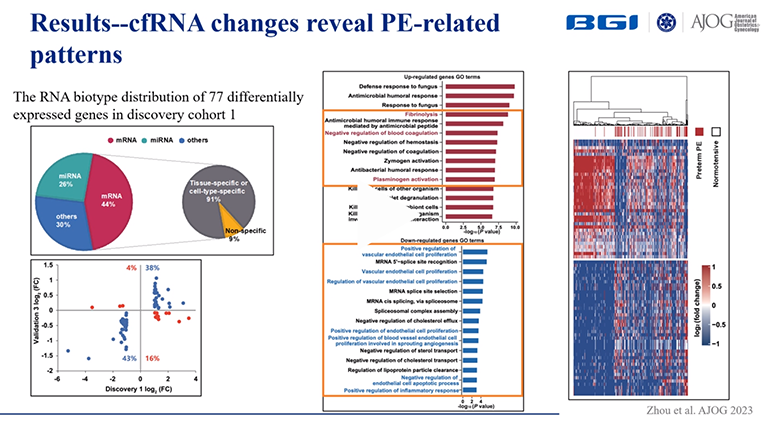
Image Credit: BGI Genomics
2. Two classifiers were developed for the prediction of preterm preeclampsia and early-onset preeclampsia before diagnosis using 13 cell-free RNA signatures and two clinical features (in vitro fertilization and mean arterial pressure), respectively.
3. For the first time, microRNA, messenger RNA, and long noncoding RNA were shown to simultaneously act as potential biomarkers of preeclampsia, showing great promise for preeclampsia prevention in the future.
4. Abnormal cell-free messenger RNA, microRNA, and long noncoding RNA molecular changes helped to elucidate the pathogenic determinants of preeclampsia.
Leveraging cfRNA for NIPT research
This study’s findings considerably expand the current knowledge in this field, demonstrating that combinations of different biotypes of RNA and two clinical features correlate with more accurate preeclampsia risk prediction.
This presents new therapeutic opportunities for the reduction of pregnancy complications and fetal morbidity.
BGI Genomics will continue to utilize multi-omics technologies to conduct research concerning maternal and infant health to provide more possibilities for prenatal testing of genetic disorders.
This project, run by BGI Genomics, adheres to relevant regulations related to biological and medical research and has received approval from the company’s Ethics Committee.
Source: BGI Genomics
References and Further Reading
-
Zhou, Si, Li, Jie, and Yang, Wenzhi. et al. 2023. "Noninvasive preeclampsia prediction using plasma cell–free RNA signatures". https://doi.org/10.1016/j.ajog.2023.05.015
About BGI Genomics
BGI Genomics is the world's leading integrated solutions provider of precision medicine, now serving customers in more than 100 countries.
They provide academic institutions, pharmaceutical companies, healthcare providers, and other organizations with integrated genomic sequencing, proteomic services, clinical testing, and solutions across a broad range of applications.
They have more than 20 years of genomics experience helping customers and partners achieve their goals by delivering rapid, high-quality results using a broad array of cost-effective, cutting-edge technologies, including their own innovative DNBSEQ™ sequencing technology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.