Sponsored Content by BGI GenomicsReviewed by Olivia FrostJul 27 2023
In December 2022, the American College of Medical Genetics and Genomics made a recommendation that all expectant mothers should be offered non-invasive prenatal testing (NIPT) instead of traditional screening methods, as stated in its Evidence-Based Clinical Practice Guideline (EBG).
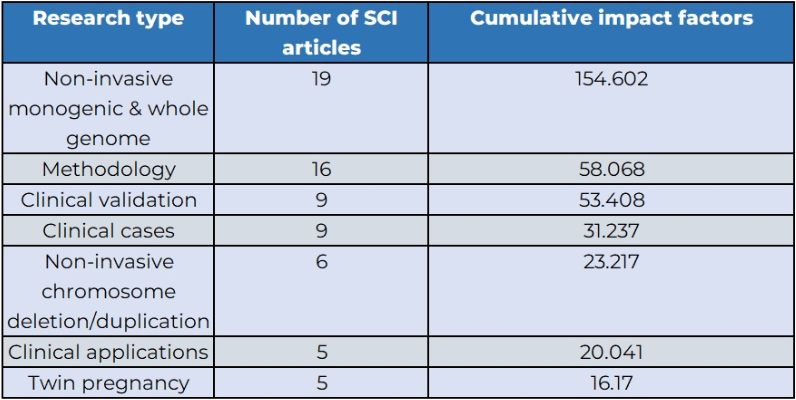
BGI Genomics has been at the forefront of providing NIPT services supported by extensive research, as evidenced by the publication of over 60 SCI papers on the topic, boasting a total impact factor exceeding 350 points.
These studies have appeared in scientific journals such as Cell, Genome Medicine, and Genetics in Medicine, among others.
Source: BGI Genomics
BGI Genomics' research covers various aspects of prenatal testing, including clinical validation, applications, cases, twin pregnancies, non-invasive chromosome deletion/duplication, and non-invasive single gene & whole genome analyses.
Considering the wealth of data available, this article seeks to provide a brief overview of three recent impactful papers.
Clinical impacts of genome-wide non-invasive prenatal testing for rare autosomal trisomy
Publication Date: October 28, 2022
Publication: American Journal of Obstetrics & Gynecology MFM

Image Credit: BGI Genomics
Research results
The utility of genome-wide NIPT in detecting several rare autosomal trisomies in pregnancies is a subject of debate due to its relatively low positive predictive value (PPV). The need for further evidence regarding the clinical application of NIPT for detecting such rare trisomies is recognized.
Researchers conducted an investigation into the origins of rare autosomal trisomies and their implications in clinical settings. From a total of 89,242 pregnancies, NIPT detected 154 cases of rare autosomal trisomies (0.17%). Clinical follow-up revealed adverse perinatal outcomes in 40% of these cases.
The ratio of NIPT-revealed placental mosaicism was significantly higher in women who experienced adverse perinatal outcomes than those who did not (0.688 vs. 0.332; P.001). These results provide valuable data that can be used to advise a patient testing positive for a rare autosomal trisomy and for the subsequent risk management of the pregnancy.
Effective identification of maternal malignancies in pregnancies undergoing non-invasive prenatal testing
Publication Date: February 10, 2022
Publication: Frontiers in Genetics

Image Credit: BGI Genomics
Research results
Maternal malignancy can cause false-positive results in NIPT tests, and currently, there is no efficient way to differentiate between maternal cancer patients and pregnant women with multiple chromosomal aneuploidies (MCA) results using NIPT. This study involved 496 patients with MCA results via NIPT between January 2016 and June 2019.
These participants were divided into two groups based on clinical follow-ups: those with cancer and those without. From January 2016 to December 2017, a cohort of 42 maternal cancer patients and 294 non-cancer cases were utilized to develop a method known as the "mean of the top five chromosomal z scores" (MTOP5Zscores).
Among the maternal cancer cases, 62 instances of breast cancer, liver cancer, and lymphoma were confirmed. The MTOP5Z scores exhibited a sensitivity of 85% and a specificity of 80% in identifying maternal cancer among pregnant women with MCA results.
For predicting breast cancer, liver cancer, and lymphoma, the classifier demonstrated sensitivities of 93.33%, 66.67%, and 50%, respectively, along with specificities of 66.67%, 90%, and 97.06%. The positive predictive values (PPV) for breast cancer, liver cancer, and lymphoma were 60.87%, 72.73%, and 80%, respectively.
Expanding the scope of non-invasive prenatal testing to detect fetal chromosomal copy number variations
Publication Date: May 12, 2021
Publication: Frontiers in Molecular Biosciences

Image Credit: BGI Genomics
Research results
While NIPT has effectively detected common fetal trisomies, its capability to identify other chromosomal abnormalities requires validation. To address this, the team investigated the positive rate in next-generation sequencing (NGS) at different read depths and developed a fetal copy number variant (CNV) detection technique in NIPT.
The positive CNV detection rate increased with higher read depths. Moreover, NGS demonstrated higher positive CNV identification rates with tiny fragments at 25 M compared to karyotype analysis. Increasing the read depth in NGS improves positive CNV detection while reducing false-positive results.
In conclusion, NIPT by NGS holds promise as a potential method for fetal CNV detection. Raising the read depth is crucial for reducing the rate of false positive CNV detection and the need for unnecessary amniocentesis for suspected CNVs.
About BGI Genomics
BGI Genomics is the world's leading integrated solutions provider of precision medicine, now serving customers in more than 100 countries.
They provide academic institutions, pharmaceutical companies, healthcare providers, and other organizations with integrated genomic sequencing, proteomic services, clinical testing, and solutions across a broad range of applications.
They have more than 20 years of genomics experience helping customers and partners achieve their goals by delivering rapid, high-quality results using a broad array of cost-effective, cutting-edge technologies, including their own innovative DNBSEQ™ sequencing technology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.