Sponsored Content by TissueGnosticsReviewed by Maria OsipovaJun 12 2023
Utilizing TissueFAXS whole-slide imaging and the HistoQuest single-cell analysis solution, a recent study has unveiled the potential of xCT inhibition, induced by erastin, as a promising therapeutic strategy for addressing HNSCC tumorous cells through the initiation of cell ferroptosis.
Head and neck squamous cell carcinoma (HNSCC) refers to head and neck cancers originating from the mucosal epithelium in the oral cavity, larynx, and pharynx, posing a significant global health burden. With HNSCC being the sixth most prevalent cancer worldwide, its impact is profoundly negative.1
In 2020 alone, approximately 377,700 new cases were reported, resulting in 177,800 deaths.2 Moreover, HNSCC cases are predicted to increase by 30% by 2030, amounting to 1.08 million new cases annually.1
Given the unfavorable clinical outcomes experienced by many HNSCC patients, developing innovative therapeutic approaches is imperative.
Despite advancements in HNSCC therapeutics, treatment methods for this disease have remained unchanged over the past few decades.1
Therefore, there is a pressing need for novel therapeutic strategies to enhance survival rates and treatment outcomes for HNSCC patients. A critical factor in improving survival rates is increasing the number of patients responding positively to therapy.
However, due to the highly heterogeneous nature of HNSCC tumor cells, differences in survival rates can also be influenced by factors such as the anatomical site, cancer stage, and the presence of human papillomavirus (HPV) infection.3
HNSCC tumors encompass diverse cell populations that interact through signaling pathways, making it crucial to ascertain their quantity and spatial distribution for comprehensive study.
Localization and analysis of subpopulations of tumor cells can be achieved by examining the expression of biomarkers like xCT, which can be automatically imaged using the TissueFAXS platform and quantified through the application of HistoQuest (TissueGnostics) single-cell analysis software.
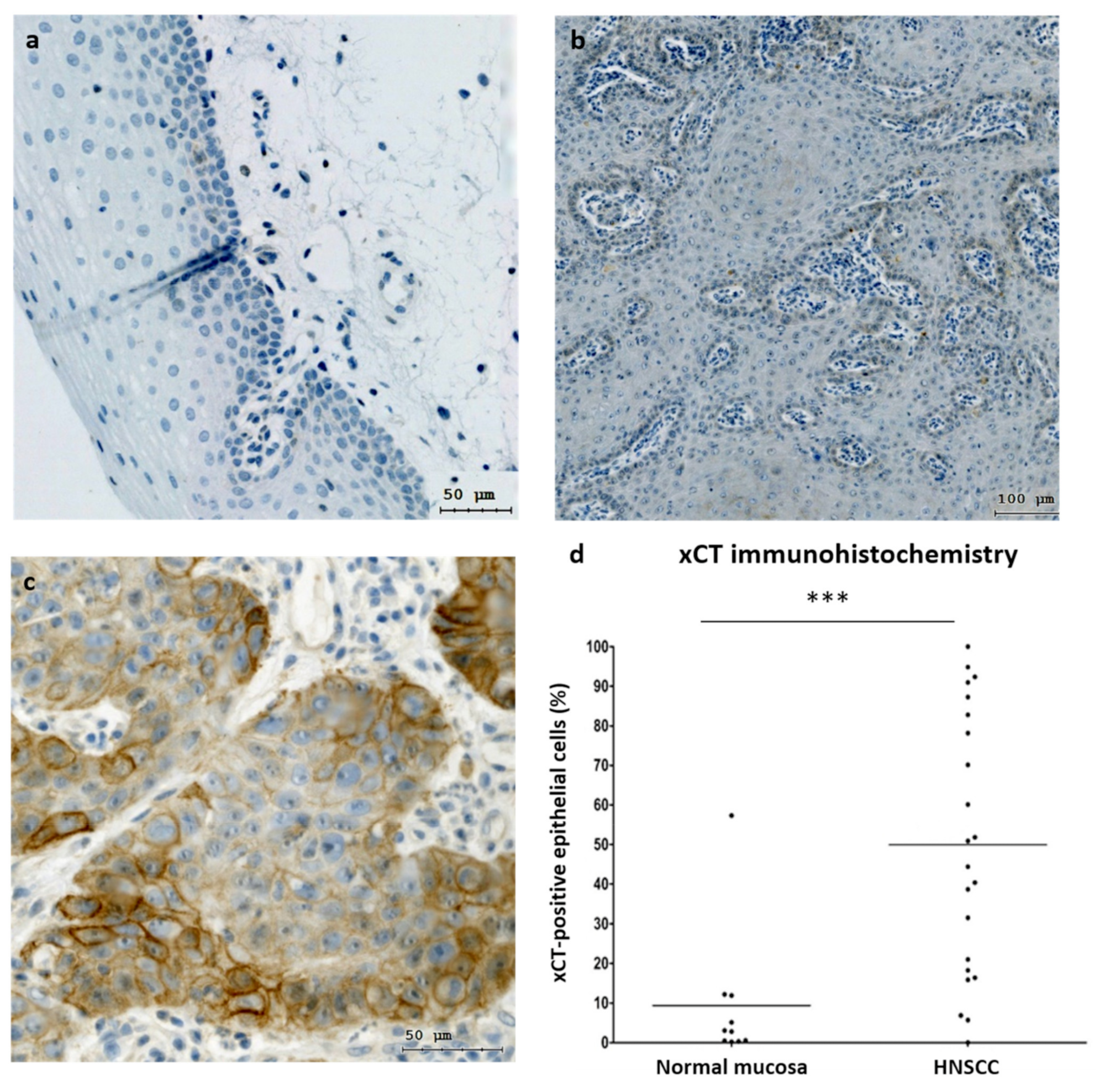
Image Credit: Savic, D., Steinbichler, T.B., Ingruber, J., Negro, G., Aschenbrenner, B., Riechelmann, H., Ganswindt, U., Skvortsov, S., Dudás, J. and Skvortsova, I.I. 2023. Erk1/2-Dependent HNSCC Cell Susceptibility to Erastin-Induced Ferroptosis. Cells. 12(2), p.336.
Ferroptosis cell death
Over the past decade, researchers have identified a distinctive form of iron-dependent cell death known as ferroptosis. The cell death is triggered by erastin, a compound that hinders cystine uptake through the cystine/glutamate antiporter system xCT, consequently impeding the cell's antioxidant defense mechanism.4 As a result, an iron intake escalation, as observed in HNSCC cells, leads to intracellular augmentation.
While further research is necessary to understand the ferroptotic pathway, inhibiting xCT can potentially eliminate cancer cells that exhibit resistance to HNSCC chemo-radiotherapy, positioning it as a viable treatment target.3
Concurrently, an elevation of reactive oxygen species (ROS) occurs within the cell during this process, prompting cells to potentially upregulate their antioxidant system in response.
Several MAP kinases, including Erk1/2, play a role in regulating oxidative stress within the cell, and inhibiting these kinases has proven to safeguard cells against ferroptosis.5
Therefore, targeting xCT regulation through Erk1/2 could be a promising therapeutic approach for anti-tumor therapies.
Dr. Dragana Savic and her team initially recognized this potential by investigating whether the activation of Erk1/2 rendered HNSCC cells more susceptible to ferroptosis and if the expression and phosphorylation of Erk1/2 could be modulated through erastin to manipulate ferroptosis development.
Using TissueFAXS and HistoQuest to assess xCT regulation by Erk1/2
The study investigated the correlation between xCT activity, encoded by SLC7A11, within cells and patient survival rates to evaluate its potential as a therapeutic target for HNSCC patients.
To achieve this, TissueFAXS was employed for the automated scanning of tissue slides stained with hematoxylin and xCT, while HistoQuest was utilized to quantify xCT expression at the single-cell level based on signal staining intensity.
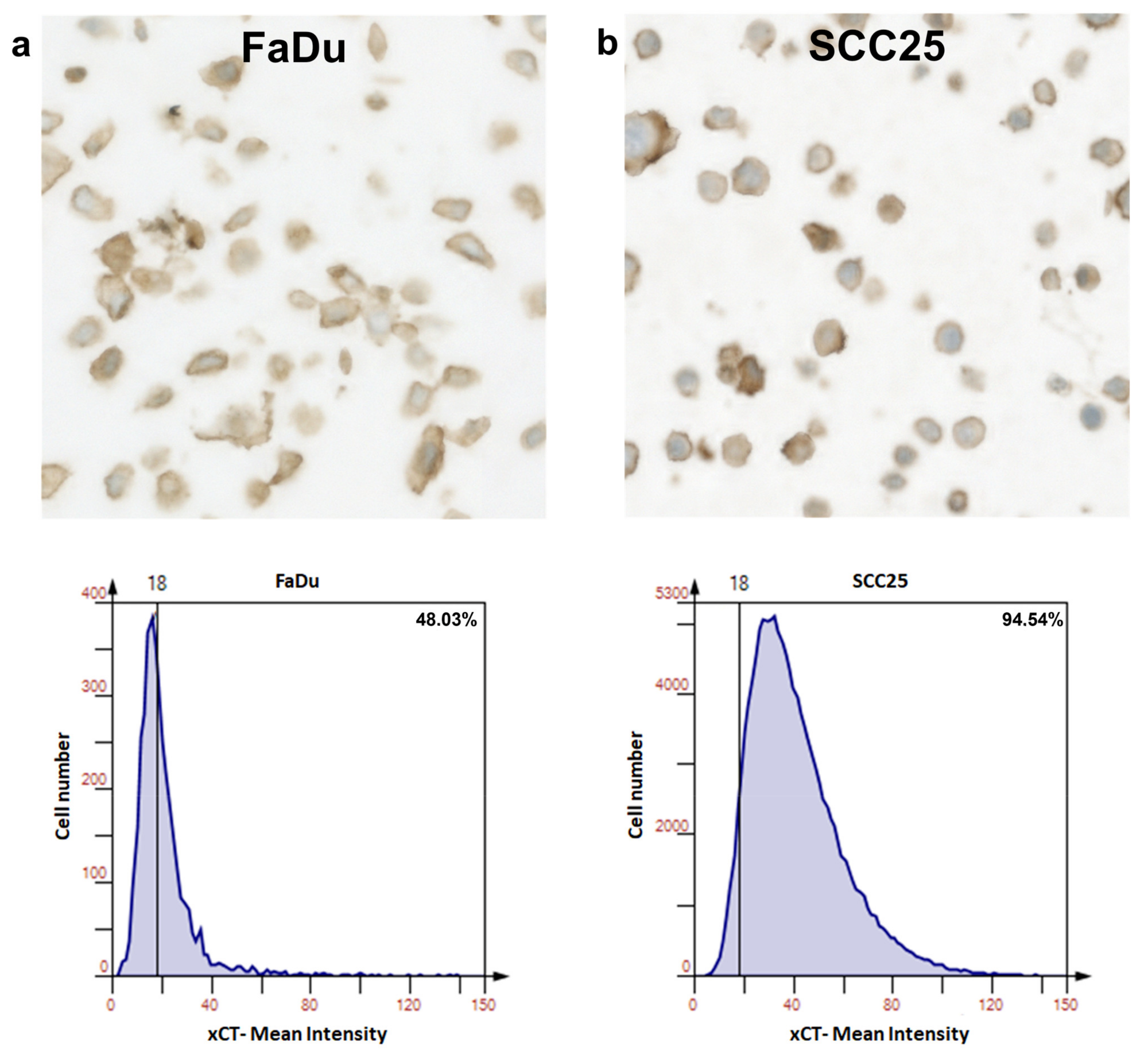
Image Credit: Savic, D., Steinbichler, T.B., Ingruber, J., Negro, G., Aschenbrenner, B., Riechelmann, H., Ganswindt, U., Skvortsov, S., Dudás, J. and Skvortsova, I.I. 2023. Erk1/2-Dependent HNSCC Cell Susceptibility to Erastin-Induced Ferroptosis. Cells. 12(2), p.336.
Cellular recognition was based on nuclear hematoxylin staining, and cells positive for the marker were expected to exhibit xCT expression surpassing a predefined threshold.
HistoQuest software quantified the number of positive cells relative to negative cells, considering staining intensity, to assess the impact of Erk1/2 activation on cell ferroptosis.
This evaluation was conducted on both normal mucosa and HNSCC samples to compare the differences in xCT expression. Subsequently, tumor cells were treated with erastin to investigate the role of Erk1/2 in reducing the viability of cells overexpressing xCT.
The study revealed that HNSCC patients with elevated cellular levels of xCT experienced significantly lower survival rates compared to those with lower levels.
The xCT molecule was identified as a crucial negative regulator of ferroptosis, indicating its potential as a target in HNSCC treatment.3 These findings align with previous studies reporting reduced overall survival in oncology patients with xCT overexpression.6,7
Inhibiting the Erk1/2 signaling pathway resulted in decreased efficacy of erastin due to its impact on ROS production. Therefore, it is suggested that carcinoma cells expressing phosphorylated Erk1/2 exhibit heightened sensitivity to ferroptosis.3
Consequently, cellular levels of phosphorylated Erk1/2 can be considered as predictive markers for the response of cancerous cells to erastin, enhancing current oncology treatments.
TissueFAXS and HistoQuest represent cutting-edge immunohistochemistry technologies that effectively assess potential therapeutic targets and predictive molecules/pathways, advancing oncology treatment and improving patient outcomes and survival rates.
References and further reading
- Johnson, D.E., Burtness, B., Leemans, C.R., Lui, V.W.Y., Bauman, J.E. and Grandis, J.R. 2020. Head and neck squamous cell carcinoma. Nature reviews Disease primers. 6(1), p.92.
- Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A. and Bray, F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 71(3), pp.209-249.
- Savic, D., Steinbichler, T.B., Ingruber, J., Negro, G., Aschenbrenner, B., Riechelmann, H., Ganswindt, U., Skvortsov, S., Dudás, J. and Skvortsova, I.I. 2023. Erk1/2-Dependent HNSCC Cell Susceptibility to Erastin-Induced Ferroptosis. Cells. 12(2), p.336.
- Dixon, S.J., Lemberg, K.M., Lamprecht, M.R., Skouta, R., Zaitsev, E.M., Gleason, C.E., Patel, D.N., Bauer, A.J., Cantley, A.M., Yang, W.S. and Morrison, B. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149(5), pp.1060-1072.
- Poursaitidis, I., Wang, X., Crighton, T., Labuschagne, C., Mason, D., Cramer, S.L., Triplett, K., Roy, R., Pardo, O.E., Seckl, M.J. and Rowlinson, S.W. 2017. Oncogene-selective sensitivity to synchronous cell death following modulation of the amino acid nutrient cystine. Cell reports. 18(11), pp.2547-2556.
- Shi, Z.Z., Tao, H., Fan, Z.W., Song, S.J. and Bai, J. 2021. Prognostic and immunological role of key genes of ferroptosis in pan-cancer. Frontiers in Cell and Developmental Biology. 9, p.748925.
- Lee, J.R., Roh, J.L., Lee, S.M., Park, Y., Cho, K.J., Choi, S.H., Nam, S.Y. and Kim, S.Y. 2018. Overexpression of cysteine‐glutamate transporter and CD44 for prediction of recurrence and survival in patients with oral cavity squamous cell carcinoma. Head & neck. 40(11), pp.2340-2346.

About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences and technical microscopy samples.
TG was founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA and China and customers in 28 countries.
TG systems offer integrated workflows, i.e. scan and analysis, for digital slides or images of tissue sections, Tissue Microarrays (TMA), cell culture monolayers, smears and of other samples on slides and oversized slides, in Microtiter plates, Petri dishes and specialized sample containers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.