Sponsored Content by TissueGnosticsReviewed by Maria OsipovaApr 24 2025
Recent research has leveraged advanced spatial biology solutions to explore the role of the B7-H4 ligand in lung cancer.
Lung cancer continues to be the leading cause of cancer-related deaths around the world despite advances in treatment options such as immunotherapy and other targeted therapies.1
This cancer retains a high fatality rate due to a combination of early metastatic development and late symptom presentation, with over 50% of cases diagnosed at stage four and a 5-year survival rate of around 20%.1,2
Immune checkpoint inhibitors (ICIs) and other immunotherapies aim to eradicate tumors by promoting antitumor immune responses within the body, but treating these ‘immune-cold’ tumors has proven to be a challenge in immunotherapies.3 This is because these types of tumors exhibit a lack of T-lymphocyte infiltration and pre-existing antitumor immune response.3,4
Research has highlighted links between immune-cold tumors and elevated levels of the B7-H4 ligand. This ligand regulates T cells, macrophages, and neutrophils, and has been associated with a worse response to ICI therapy and poor prognosis in a range of cancers.4,5 B7-H4 is known to undergo posttranscriptional modification, but there have been limited studies into this phenomenon.4,5
Ubiquitination is a key example of a posttranscriptional modification that the protein may undergo.5 This posttranscriptional modification involves ligating the target protein to ubiquitin, tagging the protein for degradation in the proteasome.5
Ubiquitination is offset by deubiquitination, which removes ubiquitin from target proteins.5,6 Target protein activity is regulated in almost all aspects of biological activity via a balance of these two processes, including DNA repair, the cell cycle, and apoptosis.5
Research has highlighted that dysregulation of ubiquitination and deubiquitination is evident in several cancers.6
Ubiquitin-specific protease 2a (USP2a) is a deubiquitinating enzyme linked to aggressive tumor types, including proliferation, migration, and invasion. Researchers from China’s Soochow University in Suzhou looked into whether USP2a plays a role in tumor immunity in lung cancers.5
Promoting B7-H4 stability
Lung cancer cell lines with EGFR activating mutations (EGFR MT) were investigated because they have been linked to both B7-H4 upregulation and especially poor responses to immunotherapy.6,7
The team initially demonstrated that proteasome inhibition increases B7-H4 levels, confirming that B7-H4 is degraded in the proteasomes. They also showed that EGFR MT considerably extends the half-life of B7-H4, highlighting that silencing EGFR expression limits B7-H4 half-life. These findings indicate that EGFR MT helps to protect B7-H4 from degradation.
It was also shown that transfecting EGFR-activating mutations into cell lines results in EGFR MT inhibiting B7-H4 ubiquitination, suggesting that this is the mechanism by which these mutations promote B7-H4 stability.6
The team studied the effect of USP2a on B7-H4 and its ubiquitination in the absence of EGFR to rule out the possibility that an increase in B7-H4 induced by USP2a is an indirect effect of USP2a on EGFR degradation.
EGFR depletion did not prevent USP2a from upregulating B7-H4 levels or deubiquitinating the protein. However, USP2a depletion reduced EGFR MT-induced B7-H4 stability.
These findings indicate that USP2a directly regulates the stability of B7-H4 and mediates the upregulation of B7-H4 induced by EGFR-MT, independent of any potential effects on EGFR degradation.
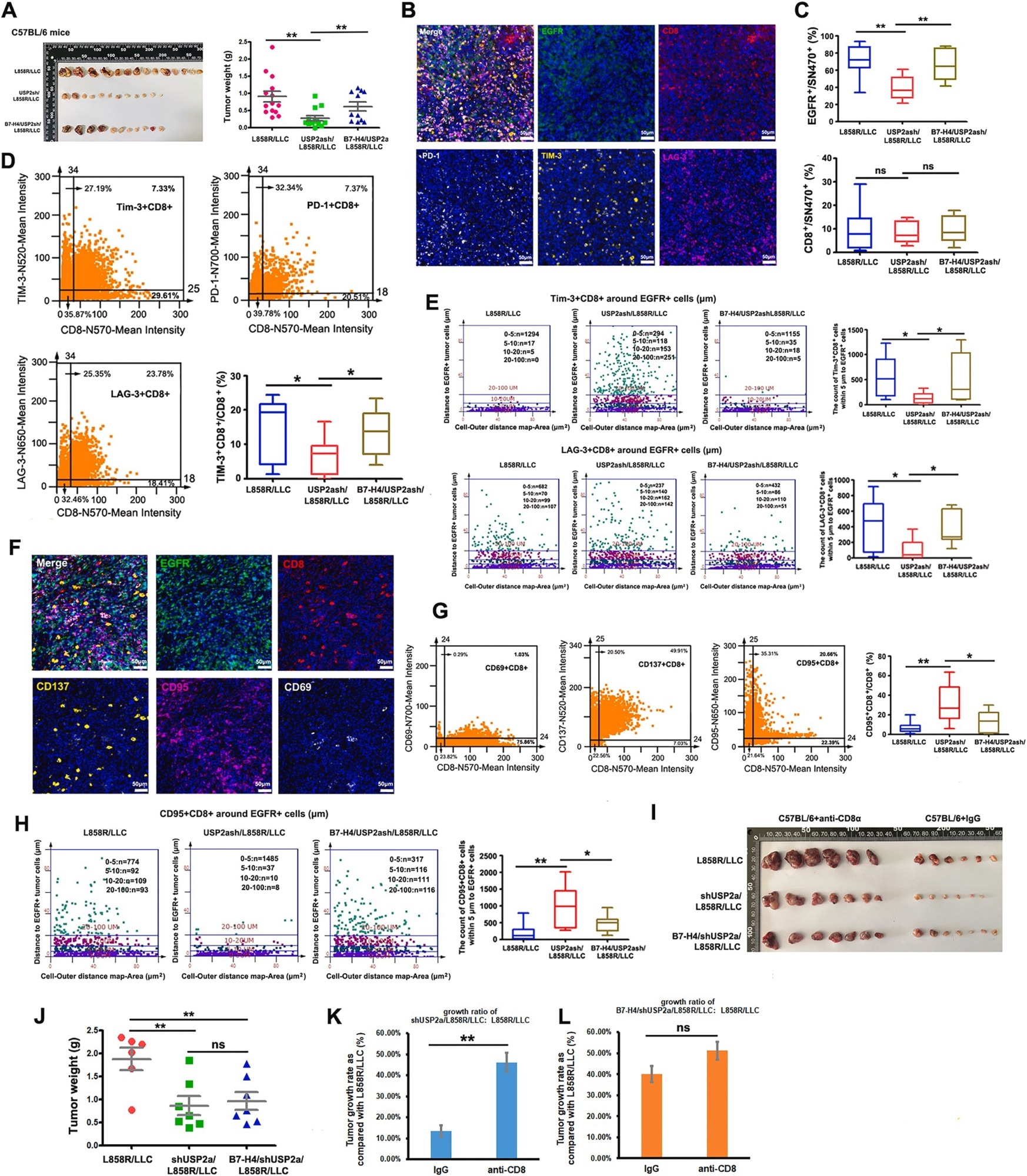
From Figure 7. USP2a contributes to tumor immunosuppression in immune-competent C57BL/6 mice and B7–H4 is involved in this process. Image Credit: Youwei Lu, Yu Sun, Jie Zhang, Miao Kong, Zhiming Zhao, Boshu Sun, Yuan Wang, Ying Jiang, Shaomu Chen, Chao Wang, Yin Tong, Liangzhu Wen, Moli Huang, Fengying Wu, Liang Zhang. The deubiquitinase USP2a promotes tumor immunosuppression by stabilizing immune checkpoint B7–H4 in lung adenocarcinoma harboring EGFR-activating mutants, Cancer Letters.
Revealing associations
The researchers confirmed that USP2a expression is increased in EGFR-MT lung adenocarcinoma. This was confirmed by studying mRNA expression and applying immunohistochemical (IHC) staining to clinical lung adenocarcinoma samples.
IHC staining revealed a positive association between USP2a protein expression and B7-H4 protein expression in human lung adenocarcinoma.
USP2a level and EGFR MT were also found to correlate with the infiltration of certain immune cells, including CD8+ T cells. It was noted, however, that these had no relationship with the infiltration of other types of immune cells, highlighting the role of the USP2a-B7-H4 axis in immunosuppression in EGFR-MT lung cancer.6
The team studied the role of USP2a in tumor growth in immunodeficient mice to fully explore these findings. These models verified the role of USP2a in promoting tumor growth in EGFR-MT lung cancer, irrespective of B7-H4 expression.
USP2a knockdown inhibited tumor growth in immunocompetent mice, while a B7-H4 overexpression resulted in tumor growth.
Multicolor immunofluorescence revealed that knocking down USP2a increased effector T cell infiltration while decreasing exhausted T cell infiltration. B7-H4 was found to reverse this effect, showcasing its ability to mediate the role of USP2a within the tumor microenvironment.6
The researchers were featured in Cancer Letters, stating that their findings confirm the role of USP2a in promoting tumor growth and explaining that it does so by stabilizing B7-H4. They suggest that USP2a could be a useful immunotherapeutic target for EGFR-MT lung cancers.6
TissueGnostics innovation
The study presented here used TissueGnostics TissueFAXS whole-slide scanning to capture high-resolution tissue specimens and StrataQuest software to analyze protein expression.
The StrataQuest software was key to facilitating the spatial analysis of effector and exhausted T cells with respect to tumor cells.
Both tools are ideally suited to undertaking spatial analysis in cancer research. For example, TissueFAXS allows researchers to perform automated whole-slide multispectral imaging of up to 120 standard-size slides and up to eight markers in a single run.
StrataQuest allows researchers to automatically detect and quantify cells and biomarkers in tissue sections of any origin. It provides a robust and user-friendly combination of statistical analysis and integration with other bioinformatics tools.
References and further reading
- Sun, Y., et al. (2023). The significance of the crosstalk between ubiquitination or deubiquitination and ncRNAs in non-small cell lung cancer. Frontiers in Oncology, 12. https://doi.org/10.3389/fonc.2022.969032.
- Ruano-Raviña, A., et al. (2020). Lung cancer symptoms at diagnosis: results of a nationwide registry study. ESMO Open, (online) 5(6), p.e001021. https://doi.org/10.1136/esmoopen-2020-001021.
- Wang, L., et al. (2023). Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm, (online) 4(5). https://doi.org/10.1002/mco2.343.
- Song, X., et al. (2020). Pharmacologic Suppression of B7-H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers. Cancer Discovery, 10(12), pp.1872–1893. https://doi.org/10.1158/2159-8290.cd-20-0402.
- Lu, Y., et al. (2024). The deubiquitinase USP2a promotes tumor immunosuppression by stabilizing immune checkpoint B7–H4 in lung adenocarcinoma harboring EGFR-activating mutants. Cancer Letters, 596, p.217020. https://doi.org/10.1016/j.canlet.2024.217020.
- Sun, T., Liu, Z. and Yang, Q. (2020). The role of ubiquitination and deubiquitination in cancer metabolism. Molecular Cancer, 19(1). https://doi.org/10.1186/s12943-020-01262-x.
- Lu, Y., et al. (2021). B7–H4 is increased in lung adenocarcinoma harboring EGFR-activating mutations and contributes to immunosuppression. Oncogene, 41(5), pp.704–717. https://doi.org/10.1038/s41388-021-02124-6.
Acknowledgments
Produced from materials originally authored by TissueGnostics GmbH.
About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences, and technical microscopy samples.
TG has been founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA, and China, and customers in 30 countries.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.