Sponsored Content by TissueGnosticsReviewed by Maria OsipovaApr 24 2025
A team from the Medical University of Vienna, in Austria, has successfully developed an algorithm within the StrataQuest image analysis software that can automatically detect and quantify osteoclasts.
Bone undergoes a constant process of remodeling throughout a person’s life, with bone resorbed by osteoclasts and rebuilt by osteoblasts.1,2
Diseases like osteoporosis, rheumatoid arthritis, or multiple myeloma can stem from an imbalance within these two processes,1,2 meaning that there is significant interest in compounds able to modulate the balance between osteoclasts and osteoblasts. These compounds could have the potential to treat these debilitating diseases.1
Osteoclasts are cells responsible for bone resorption. These multinucleated cells form via osteoclastogenesis, a process involving the differentiation of precursor cells 1,3
It is possible to visualize this process under light microscopy, using tartrate-resistant acid phosphatase (TRAP)-staining. This challenging but well-established protocol takes advantage of the increased levels of this compound in differentiated osteoclasts.1
The current gold standard approach to the quantification of osteoclast formation involves counting the number of TRAP-positive cells with at least three nuclei.1 This time-consuming manual process is currently carried out using image processing software, precluding large-scale experiments. It is also subject to considerable intra- and inter-user variability.1,3,4
Counting the number of osteoclasts offers valuable information, but this manual approach does not reliably provide other potentially relevant information, such as the number of cells in a culture, cell area, or the number of nuclei.4,5
These properties could support research into the impact of hormones or therapeutic agents on osteoclast biology.5
There is a clear need for automated methods able to efficiently and reliably quantify osteoclasts in culture, with these methods potentially used to screen possible therapeutic agents for bone-related diseases.1,2
This need prompted a team from the Medical University of Vienna in Austria to develop an algorithm designed to enable biologists and medical scientists to automatically detect and quantify osteoclasts via commercially available automated image analysis software.5
Origins of an algorithm
The team began developing its algorithm by establishing a staining protocol. This protocol included labeling the nuclei with DAPI and deploying an antibody against the macrophage-antigen F4/80–whose low expression is a marker for differentiation into mature osteoclasts–and staining the membrane and cytoskeleton to ensure that all cells are visible.5
Cells were fixed in formaldehyde, with images captured via the TissueGnostics TissueFAXS microscopy platform.5
The algorithm was developed using a four-stage process, beginning with illumination correction for uneven illumination potentially introduced during the image acquisition process. This could interfere with the automated analysis.
Segmentation was then performed to separate pixels into foreground and background, with thresholds set to better automate this process. Adaptive thresholding and computed local thresholds were employed for each image’s subregion.
Post-processing involved cleaning up images, removing artifacts, and removing unwanted cells. The final step labeled the remaining cells, allowing measurements to be computed directly at the level of single cells.
The algorithm was trialed via an investigation into the effect of the putative therapeutic agent melatonin on osteoclast formation in vitro.5
The algorithm in practice
A culture of murine bone marrow-derived macrophages was incubated with RANKL and M-CSF, vital for osteoclast precursor differentiation to functionally active osteoclasts.1,5 Melatonin was applied at three different concentrations (1, 0.1, and 0.01 μM) throughout the cultivation period.
The pineal gland produces the hormone melatonin. Because it has long been believed that melatonin has a beneficial effect on bone structure, it has been proposed as a potential osteoporosis therapy in pre- and post-menopausal women.
Melatonin has already been shown to have a stimulatory influence on osteoblast activity, but it has not yet been shown whether it directly affects osteoclasts.5
The team used its osteoclast detection algorithm within TissueGnostics’ StrataQuest platform to perform automated analysis. A range of parameters was measured, including the number of nuclei per cell, mean intensity values of labeled proteins, the total number of cells, and the mean cell area.
Discriminating between multinucleated cells with low F4/80 intensity (osteoclasts) and mononucleated cells with higher F4/80 intensity (precursors) showed that melatonin did not impact osteoclast number.
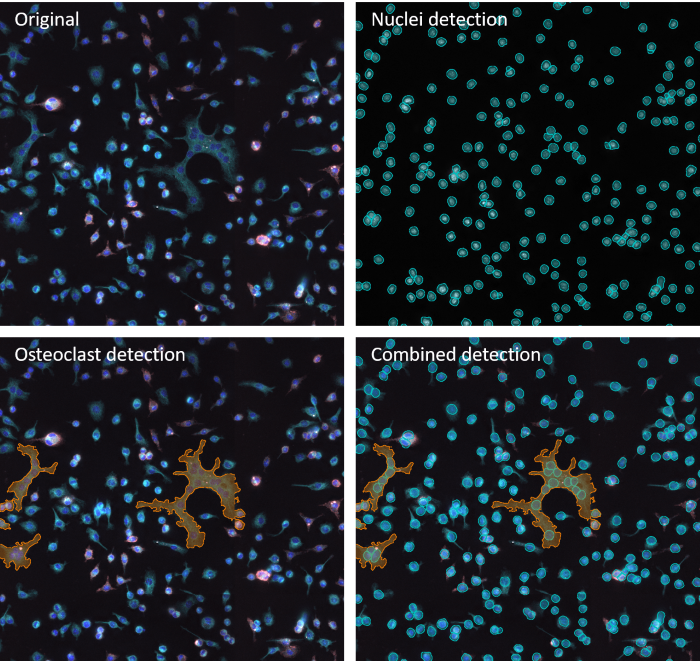
Example of image anaylsis performed by IF Cell Culture - Osteoclast App. Image Credit: TissueGnostics
However, the mean area of osteoclasts after treatment with melatonin increased 130-200% at a concentration of 10 nM, compared with a control concentration of 1 μM, where the mean number of nuclei/osteoclasts increased by up to 140%.
These effects did not occur for precursor cells, indicating that melatonin stimulated the differentiation of precursors into mature osteoclasts.5
Melatonin had not previously been shown to directly influence osteoclast activity, and this research would have been impossible to perform with the conventional manual approach.
The researchers suggest an automated staining system could be added to this process, enabling automated osteoclast quantification into a fully automated medium- to high-throughput workflow with potential use in a clinical setting.5
Integrating innovation
The StrataQuest software from TissueGnostics was central to the research. This state-of-the-art software offers a range of functionalities, including the ability to automatically detect, quantify, and analyze interactions between different cell types and structures such as colon crypts, blood vessels, and immune aggregates.
The StrataQuest software was coupled with TissueFAXS, offering a convenient workflow that could automatically acquire up to eight slides. This approach also enabled automatic image stitching, a key consideration when studying cultures containing large cells such as osteoclasts.
Since its development, the new algorithm has been incorporated into the StrataQuest software and is available as an integrated app: the IF Cell Culture - Osteoclast App. This powerful new feature allows users to perform automated nuclei segmentation to identify cultured multinucleated osteoclasts and one or two additional markers.
A range of other apps, including Safranin O, Goldner, and Von Kossa, developed for the automated analysis of commonly used stains in bone research are also available.
For example, the Bone Mineralization App can be used to separate Safranin O-stained bone tissue into its morphological substructures, including bone marrow, mineralized bone, cartilage, and mineralized cartilage.
Measurements evaluated via this app include BV (total bone volume), MCV (mineralized cartilage), TV (trabecular bone volume), CV (cartilage volume), and bone marrow (BM).
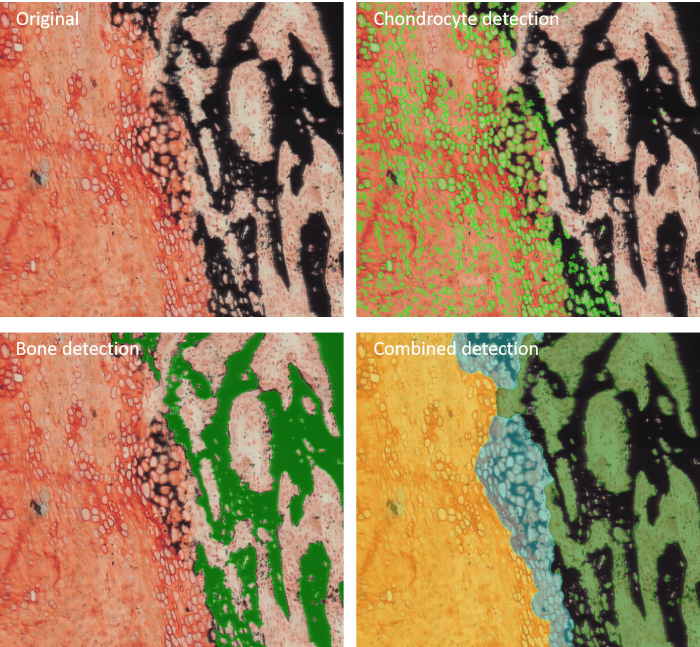
Example of image anaylsis performed by Bone Mineralization App. Image Credit: TissueGnostics
References and further reading
- Cohen-Karlik, E., et al. (2021). Quantification of Osteoclasts in Culture, Powered by Machine Learning. Frontiers in Cell and Developmental Biology, 9. https://doi.org/10.3389/fcell.2021.674710.
- Sampsa Kohtala, Vikene, M., et al. (2022). Automated Quantification of Human Osteoclasts Using Object Detection. Frontiers in Cell and Developmental Biology, 10. https://doi.org/10.3389/fcell.2022.941542.
- Davies, B.K., et al. (2023). A Machine Learning-Based Image Segmentation Method to Quantify In Vitro Osteoclast Culture Endpoints. Calcified Tissue International, 113(4), pp.437–448. https://doi.org/10.1007/s00223-023-01121-z.
- Heindl, A., et al. (2011). Automated detection, quantification and characterization of osteoclasts in cultures using a combined image-processing and machine-learning strategy. Bone, 48, pp.S125–S126. https://doi.org/10.1016/j.bone.2011.03.242.
- Heindl, A., et al. (2011). Towards the Automated Detection and Characterization of Osteoclasts in Microscopic Images. pp.27–48. https://doi.org/10.1007/978-3-7091-0520-7_2.
Acknowledgments
Produced from materials originally authored by TissueGnostics GmbH.
About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences, and technical microscopy samples.
TG has been founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA, and China, and customers in 30 countries.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.