Sponsored Content by MerckReviewed by Emily MageeFeb 1 2024
Three-dimensional printing (3DP) can be used to generate intricate tissue engineering scaffolds based on computer designs derived from patient-specific anatomical data.
 Image Credit: everytime/Shutterstock.com
Image Credit: everytime/Shutterstock.com
Initially utilized in the biomedical sector for crafting pre-surgical visualization models and molds for tools, 3DP has evolved to enable the production of tissue engineering scaffolds, tissue analogs, and organs-on-chip for diagnostic purposes.
The recent surge in public interest and the accessibility of affordable printers has sparked a renewed interest in merging stem cell technology with custom three-dimensional (3D) scaffolds, giving rise to the domain of personalized regenerative medicine.
However, several technological challenges must be addressed before 3DP can become a standard practice for regenerating complex tissues like bone, cartilage, muscles, blood vessels, or intricate 3D microarchitecture organs such as the liver or lymphoid organs.
This article will delve into the technological advancements contributing to the progress of 3DP in tissue engineering scaffolds, the current materials employed for crafting 3DP scaffolds, and the persisting challenges.
Most 3DP methods employ a layer-by-layer process for object fabrication. The general 3DP procedure involves:
- Creating a solid 3D computer model, either from medical imaging data or through computer-aided design (CAD).
- Slicing the 3D model into consecutive two-dimensional (2D) slices.
- Constructing each slice through a computer-controlled layer-by-layer process.
- Concluding with post-processing, such as surface modification, to form nanoarchitecture.
This approach reduces intricate 3D features like internal voids, cantilevers, undercuts, and narrow, tortuous paths to a stack of common 2D features like circles, lines, and points.
Exempted from tooling path restrictions, where material removal is limited to physically accessible areas, these additive technologies offer significantly greater shape complexity.
Creating these complex 3D shapes is highly beneficial in biomedical engineering, and various 3DP techniques have already been introduced to fabricate objects, such as controlled microarchitecture and microstructures for application in biomedical and tissue engineering.
Biomedical researchers are swiftly adopting 3DP due to its unique blend of form freedom and material deposition technology. This combination allows for exceptional control over the three key elements of tissue engineering: cells, signals, and scaffolding substrates.
The expiration of core 3D printing patents, along with the accessibility of budget-friendly computational power for processing extensive 3D files, has substantially fueled the recent surge in 3DP growth.
Current technological strides allow for the creation of intricate 3D structures with patient-specific macro- and microarchitecture. FreeCAD open-source software and various CAD applications permit the design and sharing of components, even within home settings.
Presently, 3D image acquisition can be accomplished using low-cost, high-resolution 3D scanners, with smartphone apps in development that promise 3D scanning capabilities.
These advancements, when combined, have expanded the popularity and availability of 3DP to a broader audience. Consumer-oriented 3DP machines can be adapted for crafting tissue scaffolds.
The widespread accessibility of quality instrumentation now addresses a key limitation of 3DP—the shortage of printable implantable biomaterials. The following sections will provide a brief introduction to each 3DP technology, along with a discussion of the materials currently employed.
Powder 3D printing
Originating from the Massachusetts Institute of Technology, powder 3D printers employ an inkjet to apply a liquid binder solution onto a powder bed for crafting 3D structures. The process initiates with the even distribution of fine powder material across the piston.
The X-Y positioning system and printhead coordinate to print the desired 2D pattern by selectively depositing binder droplets onto the powder layer. Following the printing process, the piston, powder bed, and the freshly printed part are all lowered. Subsequently, a new layer of powder is spread (Figure 1).
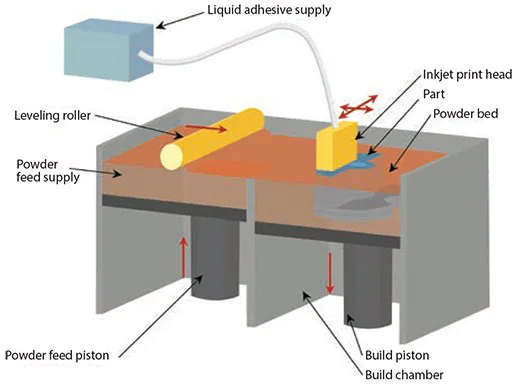
Figure 1. Illustration of powder 3D printing based on a series of steps that use a binding agent to secure powder in a 2D pattern, where the loose powder is removed after the part is printed. Image Credit: Merck
The repetition of the drop-spread-print cycle continues until the entire part is finished. The removal of unbound powder exposes the crafted part.
Biomaterials like peptides, proteins (e.g., fibrinogen, collagen), polysaccharides (e.g., hyaluronan, alginate), DNA plasmids, and living cells can be incorporated into the binder or processed as powder for direct 3D printing.
Previously used materials encompass synthetic polymers [e.g., poly (ε-caprolactone), polylactide–coglycolide or poly(l-lactic acid)] with an organic solvent as the binder and natural polymer powders [(e.g., starch (Prod. No. 03967), dextran, and gelatin(Prod. No. G1890)] with water as a binder.
Presently employed materials include ceramics [(e.g., tricalcium phosphate, hydroxyapatite(Prod. No. 289396), and calcium polyphosphate)], synthetic polymers (e.g., polyvinyl alcohol, PLGA, PCL), and natural polymers [(e.g., collagen and chitosan (Prod. Nos. 448869, 448877, and 419419)].
To use these biomaterials, the biomaterials do not usually need to be modified or functionalized in any way, but they must be in a powder form. In vivo studies have been conducted using these materials for addressing bone defects (calvarial, tibia, femoral) in rabbit, rat, and mouse models.4-9
As an alternative approach, porogens like sucrose (Prod. No. S7903), lactose, or table salt can be utilized to print the desired shape. Once polymer solutions are introduced into the interstitial spaces, dissolving these porogens results in the formation of a 3D biopolymer part.10
Fused-deposition modeling
Fused deposition modeling (FDM) involves depositing molten thermoplastic materials in specific patterns using two heated extrusion heads, each equipped with a small orifice. One nozzle dispenses the thermoplastic material, while the second dispenses temporary material to support cantilevers (Figure 2).
In a traditional FDM method, thermoplastic polymer melts into a semi-liquid state, and the head extrudes this material onto the build platform. Key material selection criteria for FDM materials include heat transfer characteristics and rheology (liquid flow behavior).
To facilitate material flow for specific lay-down patterns, thermoplastics are commonly chosen for their low melting temperature. PVC, nylon, ABS, and investment casting wax have proven successful.
For bio applications, PCL is common due to its low melting temperature (~60 °C), low glass transition temperature (–60 °C), and high thermal stability.
Other materials, such as PLGA, TCP (combined with a synthetic polymer), PMMA (Prod. Nos. 200336, 182230, and 445746), poly(ethylene glycol) terephthalate, and poly(butylene) terephthalate, are also utilized. Combinations of these materials can also be used.
This technology has been applied in numerous in vivo studies, including animal models (e.g., murine models for wound healing and rabbit bone defects)12–14 and in the treatment of a craniofacial defect in a human patient.15
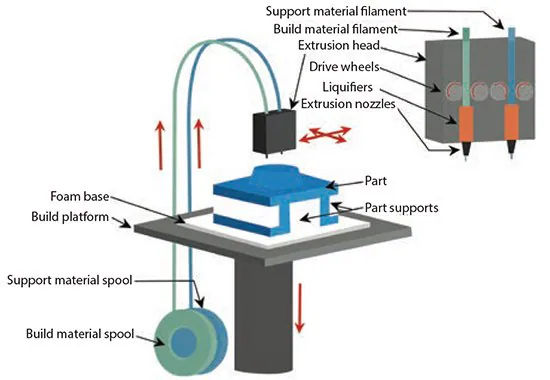
Figure 2. Illustration showing the fused-deposition modeling process, where molten thermoplastic material is deposited in specific patterns. Image Credit: Merck
Stereolithography
Stereolithography (SLA), developed in the late 1980s, is recognized as the earliest rapid prototyping process.16 The original SLA method uses a rastered HeCd-laser beam to control the polymerization of a photocurable resin in 2D patterns.17
After curing each layer, the platform with the cured structure is lowered (bottom-up approach), and another layer of uncured liquid resin is spread on top (Figure 3). The topmost layer is ready for patterning once the resin has spread.
In the top-down approach, light is projected onto a transparent plate initially positioned near the bottom of the vessel holding the liquid resin. The build can be detached from the transparent plate for each subsequent layer.
Acrylics and epoxies are typically employed in SLA; any materials used must include photocurable moieties for photo-crosslinking. For tissue engineering applications, very few biodegradable and biocompatible biomaterials are dimensionally stable enough during photo-polymerization for use in SLA.
In recent years, a growing number of polymers have been synthesized, incorporating aliphatic polyesters to facilitate biodegradation. These polymers are then modified into macromers and acrylated to enable photocrosslinking, as seen in substances like poly(ethylene glycol) diacrylate.
The expanding range of available resins with biodegradable components has enhanced the potential for encapsulating cells during processing.
These innovative macromers include segments such as PCL (polycaprolactone) or poly(d,l-lactide) and PLLA (poly-L-lactic acid) resins with modified end groups for photocrosslinking capabilities, along with PPF-DEF (poly(propylene fumarate)-diethyl fumarate).
In vivo studies have also demonstrated the ability of these materials to promote bone formation in rat cranial defects.18
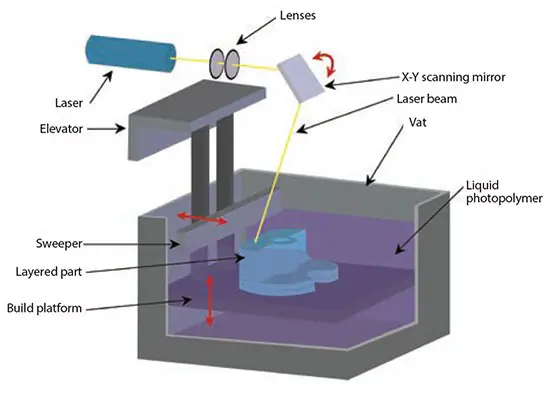
Figure 3. Illustration of the stereolithography process using layers of liquid photopolymerizable polymers. Image Credit: Merck
Selective laser sintering
In 1989,19 Selective Laser Sintering (SLS) was introduced, a process similar to powder-based 3D printing (3DP), where powder particles are fused together in thin layers.
However, SLS uniquely employs a CO2 laser beam to sinter the material. This laser scans the surface of powdered polymer particles, following a specific 2D pattern, 20 to sinter them above their glass transition temperature (Figure 4).
During the sintering process, molecular diffusion on the outermost surfaces of the particles leads to the formation of necks between adjacent particles.
After creating one layer, the part-containing piston descends, and a fresh layer of powder material is rolled across the top surface. The subsequent layer is formed and bound to the previous one. The unbound powder is removed post-fabrication, and the part undergoes heat treatment for full density.
Commonly used powder combinations include PCL and hyaluronic acid. Biomedical applications of this technique encompass bone and vascular tissue engineering,21 as well as inter-body cages for spinal fusions.22
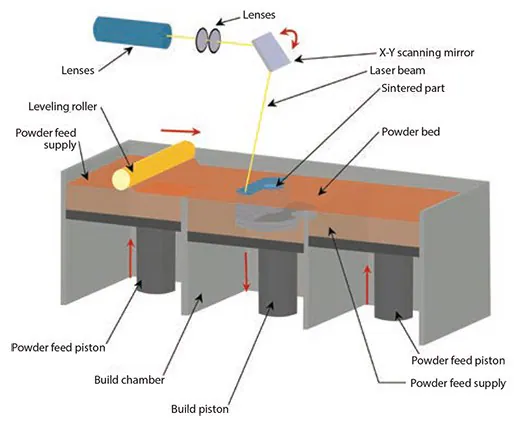
Figure 4. Illustration showing the selective laser sintering process. Image Credit: Merck
Three-dimensional plotting/direct-write bioprinting
In 2000, the Freiburg Materials Research Center developed three-dimensional plotting to fabricate soft tissue scaffolds. This technique involves extruding a viscous liquid (typically a solution, paste, or dispersion) from a pressurized syringe into a liquid medium of matching density.
The material is dispensed in either a continuous strand or individual dots from a nozzle or syringe to form a desired 3D shape using ceramics, polymers, or hydrogels.23
Likewise, bioprinting involves constructing hydrogel structures while directly incorporating cells. This technology allows for a controlled spatial distribution of cells or growth factors within scaffold structures. Materials for bioplotting include synthetic polymers and natural polymers like collagen, chitosan, alginate, agarose, or gelatin.
Most hydrogels suitable for cell bioprinting are ideal for implantation in biological environments with lower mechanical property requirements, such as cavalarial defects.24 Bioprinting has also been utilized to produce personalized cell-based chips for assessing patient-specific drug response and cytotoxicity.25
Future directions
Despite considerable progress in tissue engineering over the past five years, achieving innervation and vascularization (the development of nerves and blood vessels within these tissues) in emerging tissues remains highly challenging.
To create a fully functional 3D-printed tissue integrated with circulatory and neural control systems, functional gradients of cells and biochemical molecules must be established, mimicking conditions during embryogenesis and wound healing.
Furthermore, for cell deposition, the materials and processing conditions must be entirely compatible with biomaterials. This requirement excludes processing conditions involving UV light, heat, organic solvents, or cytotoxic photoinitiators, among others.
The integration of biochemical molecules calls for the development of methods for sustained growth factor release with controlled spatial-temporal release profiles. To advance the field, the innovative efforts currently propelling 3D printing (3DP) must focus on overcoming these challenges.
The prospects of 3D printing for tissue engineering are highly promising. Technological advancements have increased the accessibility of 3D printing beyond industry and academia, facilitating more robust design and fabrication of scaffolds.
Continuous advancements in materials and methods aim to broaden the applications of biocompatible scaffolds, such as using DNA sequences for placing various cell types in 3D to create organoid-like tissues (DNA-programmed assembly of cells or DPAC).
This convergence is pivotal in accelerating the development of 3D printing for the creation of next-generation tissue engineering structures.
References
- Cima MJ, Sachs E, Cima LG, Yoo J, Khanuja S, Borland SW, Wu B, Giordano RA. 1994. In Solid Freeform Fabr. Symp. Proc. DTIC Document,181–190.
- GRIFFITH LG, WU B, CIMA MJ, POWERS MJ, CHAIGNAUD B, VACANTI JP. In Vitro Organogenesis of Liver Tissuea. 831(1):382-397. https://doi.org/10.1111/j.1749-6632.1997.tb52212.x
- Wu BM, Borland SW, Giordano RA, Cima LG, Sachs EM, Cima MJ. 1996. Solid free-form fabrication of drug delivery devices. Journal of Controlled Release. 40(1-2):77-87. https://doi.org/10.1016/0168-3659(95)00173-5
- Abarrategi A, Moreno-Vicente C, Martínez-Vázquez FJ, Civantos A, Ramos V, Sanz-Casado JV, Martínez-Corriá R, Perera FH, Mulero F, Miranda P, et al. Biological Properties of Solid Free Form Designed Ceramic Scaffolds with BMP-2: In Vitro and In Vivo Evaluation. PLoS ONE. 7(3):e34117. https://doi.org/10.1371/journal.pone.0034117
- Tamimi F, Torres J, Gbureck U, Lopez-Cabarcos E, Bassett DC, Alkhraisat MH, Barralet JE. 2009. Craniofacial vertical bone augmentation: A comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials. 30(31):6318-6326. https://doi.org/10.1016/j.biomaterials.2009.07.049
- Tarafder S, Dernell WS, Bandyopadhyay A, Bose S. 2015. SrO? and MgO?doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res.. 103(3):679-690. https://doi.org/10.1002/jbm.b.33239
- Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. 2013. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med. 7(8):631-641. https://doi.org/10.1002/term.555
- Tarafder S, Davies NM, Bandyopadhyay A, Bose S. 2013. 3D printed tricalcium phosphate bone tissue engineering scaffolds: effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci.. 1(12):1250. https://doi.org/10.1039/c3bm60132c
- Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 2014. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 35(13):4026-4034. https://doi.org/10.1016/j.biomaterials.2014.01.064
- Chia HN, Wu BM. 2014. J. Biomed. Mater. Res. Part B Appl. Biomater..
- Zein I, Hutmacher DW, Tan KC, Teoh SH. 2002. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 23(4):1169-1185. https://doi.org/10.1016/s0142-9612(01)00232-0
- Teo EY, Ong S, Khoon Chong MS, Zhang Z, Lu J, Moochhala S, Ho B, Teoh S. 2011. Polycaprolactone-based fused deposition modeled mesh for delivery of antibacterial agents to infected wounds. Biomaterials. 32(1):279-287. https://doi.org/10.1016/j.biomaterials.2010.08.089
- Kim J, McBride S, Tellis B, Alvarez-Urena P, Song Y, Dean DD, Sylvia VL, Elgendy H, Ong J, Hollinger JO. 2012. Rapid-prototyped PLGA/?-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication. 4(2):025003. https://doi.org/10.1088/1758-5082/4/2/025003
- Shim J, Moon T, Yun M, Jeon Y, Jeong C, Cho D, Huh J. 2012. Stimulation of healing within a rabbit calvarial defect by a PCL/PLGA scaffold blended with TCP using solid freeform fabrication technology. J Mater Sci: Mater Med. 23(12):2993-3002. https://doi.org/10.1007/s10856-012-4761-9
- Probst FA, Hutmacher DW, Müller DF, Machens H, Schantz J. 2010. Rekonstruktion der Kalvaria durch ein präfabriziertes bioaktives Implantat. Handchir Mikrochir plast Chir. 42(06):369-373. https://doi.org/10.1055/s-0030-1248310
- Sweet W, Tsipis K. 1989. Reviews. Bulletin of the Atomic Scientists. 45(2):43-45. https://doi.org/10.1080/00963402.1989.11459653
- Fisher JP, Dean D, Mikos AG. 2002. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials. 23(22):4333-4343. https://doi.org/10.1016/s0142-9612(02)00178-3
- Lee JW, Kang KS, Lee SH, Kim J, Lee B, Cho D. 2011. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials. 32(3):744-752. https://doi.org/10.1016/j.biomaterials.2010.09.035
- Marcus HL, Barlow JW, Beaman JJ, Bourell DL. 1990. From computer to component in 15 minutes: The integrated manufacture of three-dimensional objects. JOM. 42(4):8-10. https://doi.org/10.1007/bf03220915
- Pattanayak DK, Fukuda A, Matsushita T, Takemoto M, Fujibayashi S, Sasaki K, Nishida N, Nakamura T, Kokubo T. 2011. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomaterialia. 7(3):1398-1406. https://doi.org/10.1016/j.actbio.2010.09.034
- Liao H, Lee M, Tsai W, Wang H, Lu W. 2013. J. Tissue Eng. Regen. Med..
- Kang H, Hollister SJ, La Marca F, Park P, Lin C. 2013. Porous Biodegradable Lumbar Interbody Fusion Cage Design and Fabrication Using Integrated Global-Local Topology Optimization With Laser Sintering. 135(10): https://doi.org/10.1115/1.4025102
- Landers R, Mulhaupt R. 2000. Macromol. Mater. Eng.. 282 (1), 17–21.
- Haberstroh K, Ritter K, Kuschnierz J, Bormann K, Kaps C, Carvalho C, Mülhaupt R, Sittinger M, Gellrich N. 2010. Bone repair by cell-seeded 3D-bioplotted composite scaffolds made of collagen treated tricalciumphosphate or tricalciumphosphate-chitosan-collagen hydrogel or PLGA in ovine critical-sized calvarial defects. J. Biomed. Mater. Res.. 93B(2):520-530. https://doi.org/10.1002/jbm.b.31611
- Xu F, Wu J, Wang S, Durmus NG, Gurkan UA, Demirci U. 2011. Microengineering methods for cell-based microarrays and high-throughput drug-screening applications. Biofabrication. 3(3):034101. https://doi.org/10.1088/1758-5082/3/3/034101
- Todhunter ME, Jee NY, Hughes AJ, Coyle MC, Cerchiari A, Farlow J, Garbe JC, LaBarge MA, Desai TA, Gartner ZJ. 2015. Programmed synthesis of three-dimensional tissues. Nat Methods. 12(10):975-981. https://doi.org/10.1038/nmeth.3553
About Merck
Our pursuit is progress for people everywhere. That's why we take a closer look at things, ask questions, and think ahead.
We've been around for more than 350 years, yet our majority owners are still the descendants of Friedrich Jacob Merck, the man who founded our company in Darmstadt, Germany in 1668.
From advancing gene-editing technologies and discovering unique ways to treat the most challenging diseases to enabling the intelligence of devices – the company is everywhere.
We are Merck. The only exceptions are the United States and Canada. Here we operate as EMD Serono in the Biopharma business, as MilliporeSigma in the Life Science business, and as EMD Performance Materials in the materials business.
Our life science business
We provide infinite solutions to solve the toughest problems in life science in collaboration with the global scientific community. Our tools, services, and digital platforms empower scientists and engineers at every stage, helping deliver breakthrough therapies more quickly.
Focus areas
With our three business units, we are a leading worldwide supplier of tools, high-grade chemicals, and equipment for academic labs, biotech, and biopharmaceutical manufacturers, as well as the industrial sector.
- Research Solutions provides our academic customers with the chemicals and tools needed to make scientific discovery easier and faster.
- Process Solutions provides drug manufacturers with process development expertise and technologies, such as continuous bioprocessing.
- Applied Solutions offers both testing kits and services to ensure that our food is safe to eat and our water is clean to drink.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.