The neuroscience-specific translational tools available from Charles River, including biomarkers, electrophysiology, behavioral and cognitive testing, and modern neuroimaging methods, have the potential to dramatically enhance the efficiency of selecting a lead program candidate by producing more predictive data than traditional measures.
Fine motor kinematic analysis
When determining the effects of a pharmacological treatment on motor capabilities, a proper tool should be employed. Standard motor tests, including open field or rotarod, are often insufficient at capturing any significant treatment effects on motor performance.
Fine motor kinematic analysis can detect subtle, yet very significant, variations of gait and movement details in rodents. This sensitivity offers a broad therapeutic window for potential treatment candidates and enables the assessment of pharmacological disruption of motor function. In comparison, more conventional measures usually evaluate overall motor function rather than fine movements. The clinical efficacy of traditional assays has been questioned, escalating the need for novel assays capable of greater sensitivity to subtle deficits in motor function.
In a high-precision kinematic assay, a mouse is made to walk from one end of a runway to the other, and its movement is recorded using a high-speed camera. The camera assesses multiple body parts from three angles, enabling a comprehensive analysis of the motor function. Detailed information is provided on aspects including changes in joint angles, interlimb coordination, and the trajectories and acceleration rates of selected body points in relation to each other.
Kinematic analysis of several known psychoactive compounds is more sensitive to subtle motor deficits than traditional motor assays. Additionally, a more detailed characterization of motor changes is provided by separating behavior into components.
Assay principle
For measurement, key body parts of the mouse are tagged and tracked using a high-speed camera, simultaneously recording three different views as the animal freely moves along a brightly illuminated runway. Full-body tracking is achieved with 31 markers in the tail, joints, paws, head and trunk and analysis of various trajectories is performed over several full gait cycles, defined by consecutive hind limb floor contacts.
Many parameters are determined to characterize the gait properties over each automatically detected gait cycle. Principal component analysis compacts the data, identifies correlations between original variables, and produces a small set of new, more sensitive, uncorrelated parameters. A total of over 100 parameters can be examined including general spatio-temporal parameters, inter-limb coordination, swing phase and paw trajectory, and body posture and joint angles.
Example Assay Setup
Image Credit: Charles River Laboratories
Source: Charles River Laboratories
| Assay Setup |
|
| Mice |
C57BL/BJ males |
| Cohort Size |
n=12 mice/group |
| Dosing Routes and Frequency |
p.o./i.p./s/c/
for other dosing routes, please contact your Charles River representative
QD for 1-7 days |
| Testing |
One time point testing within 24 hours post final dose |
| Deliverables |
Raw data, graphs, and stats (GraphPad Prism)
Separate parameters (90-100)
Pricipal component analysis |
Example Assay Performance
Three psychoactive compounds were used to validate the performance of C57BL/6J male mice: amphetamine, morphine, and pregabalin. Each compound produced a significant and distinct change in the gait features of the mice (see Figures 1 and 2)
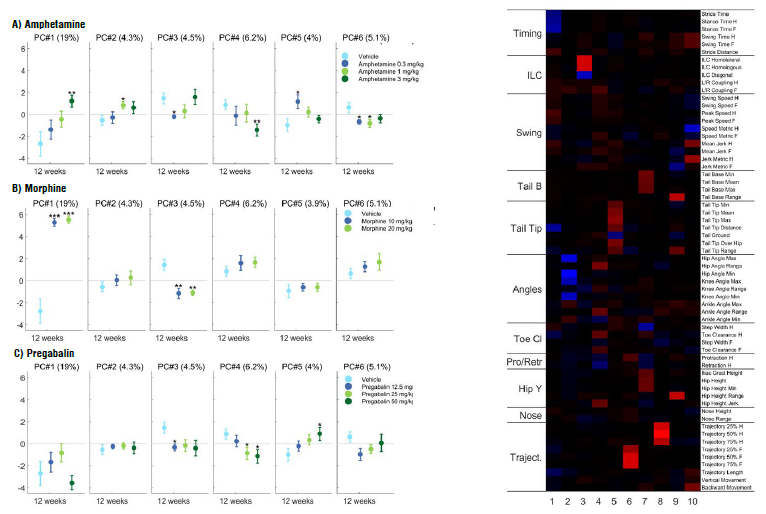
Figure 1. Fine motor and gait profiles of C57BL/6J mice treated with amphetamine (A), morphine (B) and pregabalin (C). Principal component scores (PC #1-6) on the left, and the corresponding principal component vectors (eigenvectors) shown as heat map on the right, describing mutually correlating gait parameters within each PC (red = positive correlation, blue = negative correlation). Numbers in the horizontal axis of the heat map indicate the PC number. The percentage in each panel describes the proportion of variation in the whole data set that each PC comprises. Data are presented as mean ± SEM (* p<0.05, **, p<0.01, ***p<0.001, compared to vehicle treated mice, unpaired t-test). Abbreviations: H – hind limb, F – fore limb. Image Credit: Charles River Laboratories
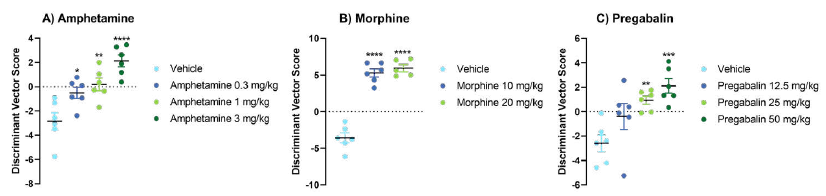
Figure 2. PCA-discriminant vector scores for different groups of C57BL/6J mice treated with amphetamine (A), morphine (B), and pregabalin (C). The score is based on differences between vehicle and drug-treated groups in all the PC scores, giving an overall fine motor kinematic score for each individual and group (*p <0.05, versus vehicle, unpaired t-test). Image Credit: Charles River Laboratories.
The most prominent changes induced by amphetamine treatment were decreased horizontal extension of the hind limbs, elevated back body posture and subtle changes in the shape of paw trajectories. Morphine treatment incuded low nose positioning, alongside very high and forward tail positioning. Pregabalin caused variations in paw trajectory and unstable acceleration of the paw during swing phase.
The results discussed in this article illustrates that fine motor kinematic analysis has the ability to differentiate subtle but significant changes induced by different pharmacological treatments, and provide further information on psychoactive treatment-induced fine motor changes in mice.
The data highlights the power of implementing a high precision kinematic assay to assess pharmacological disruption of motor function, providing a more comprehensive and sensitive measure than standard pharmacological studies. This exclusive motor test, developed by Charles River, utilizes data from spontaneous locomotion, without the need for extensive training or pharmacological induction.
About Charles River Laboratories
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work helps to improve the health and well-being of many across the globe.
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies and leading academic institutions around the globe accelerate their research and drug development efforts.
As a fully integrated partner, Charles River can support your research at any point along the drug discovery continuum.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.