This article is based on a poster originally authored by Malika Bsibsi, Abeera Popalzij, Matteo Zanella, Lieke Geerts, Mark Musters, Stefan Kostense, David Fischer, Ludo Buti, Tony Oosterveen, Ines Ferreira and Marijn Vlaming, and presented at SfN 2024
Oligodendrocytes are myelinating cells found within the central nervous system (CNS). They produce myelin, a fatty substance, which wraps around axons to assist in rapid nerve impulse conduction.
Oligodendrocytes develop from bipotential oligodendrocyte-type-2-astrocyte progenitors, which differentiate in vitro, forming either oligodendrocytes or astrocytes.
When injured, oligodendrocyte progenitor cells (OPC) react in the human adult CNS by migrating or proliferating. Oligodendrocyte dysfunction and disrupted myelin are involved in the pathogenesis of numerous diseases; it is the primary cause of symptoms in multiple sclerosis, and has also been implicated in the pathology of Alzheimer's disease.
Microglia and astrocytes have been widely characterized in their role in neurodegeneration, however oligodendrocytes have not been subject to in-depth investigation due to the relative complexity of primary oligodendrocyte isolation and culturing.
Induced pluripotent stem cell (iPSC)-derived oligodendrocytes represent a viable means of studying oligodendrocyte differentiation, maturation, and myelination, as well as investigation of their utility in models for drug discovery.
The study described here had two primary goals:
- The characterization of commercially available iPSC-derived oligodendrocytes (ioOligodendrocyte-like cells, bit.bio)
- The development of a co-culture in vitro model with iPSC-derived glutamatergic neurons (ioGlutamatergic Neurons, bit.bio) to assess the oligodendrocyte maturation and the myelination process
Methods
ioGlutamergic neurons and ioOligodendrocyte-like cells were cultured per manufacturer protocols. ioGlutamergic neurons and ioOligodendrocyte were cultured separately at day zero before detaching and resuspending ioOligodendrocyte in co-culture media comprised of BrainPhys (Stemcell) with oligodendrocyte and neuronal supporting supplements at day four.
ioOligodendrocyte-like cells resemble a pre-myelinating oligodendrocyte state when thawed, rapidly maturing from day six of culture onwards and acquiring a characteristic oligodendrocyte-like morphology featuring multiple branched processes.
Once this process was complete, cells were fixed at various times for immunocytochemistry, and DAPI, MBP, beta-III tubulin, and Neurofilament H were performed. The Yokogawa CV8000 was used to perform high-content imaging, and Arivis was used to generate an algorithm to analyze myelination and total MBP production.
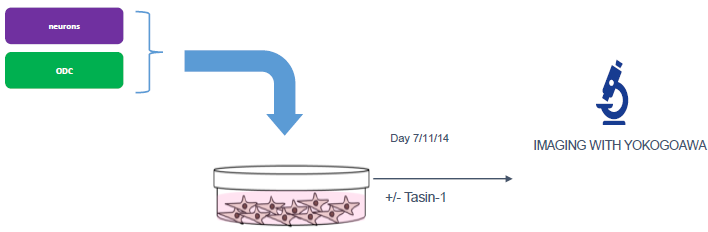
Figure 2. Schematic workflow of ioGlutamatergic neurons and ioOligodendrocyte-like cells co-culture. Neurons and oligodendrocytes were first cultured separately up to day 4. Oligodendrocytes were detached and added to neurons (Day 0 post-co-culture). Co-culture was maintained for up to day 7, day 11, or day 14 post-co-culture. Image Credit: Charles River Laboratories.
Results
MBP expression in ioOligodendrocytes-like cells monocultures
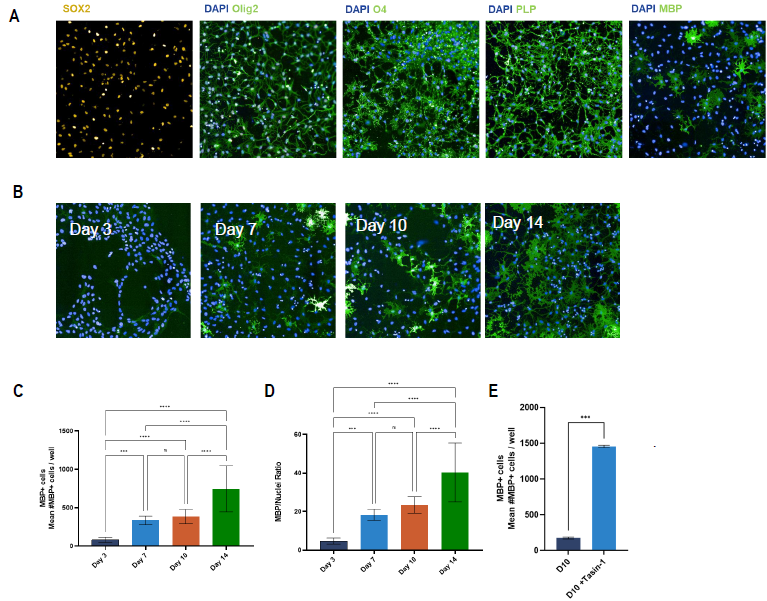
Figure 3. Characterization of ioOligodendrocyte-like cells show oligodendrocyte morphology in culture and presence of early and late-stage oligodendrocyte-specific markers. (A) Representative images of day 10 ioOligodendrocyte-like cells (20x objective) stained for different oligodendrocyte-specific markers. (B) Representative images of day 3, day 7, day 10, and day 14 post-thaw cultured stained for DAPI (nuclei) and MBP. (C-E) High-content quantification of (C) number of MBP positive (MBP+) cells, (D) MBP/Nuclei ratio at different culture time points, and (E) number of MBP+ cells with and without treatment with Tasin-1 (known inducer of MBP production) at day 10. (C-E) N=2 wells [One-way ANOVA with Tukey’s multiple comparison; *p<0.05; **p<0.005; ****p<0.0001; ns not-significant]. Image Credit: Charles River Laboratories.
MBP expression in ioOligodendrocyte + ioGlutamatergic neurons co-cultures
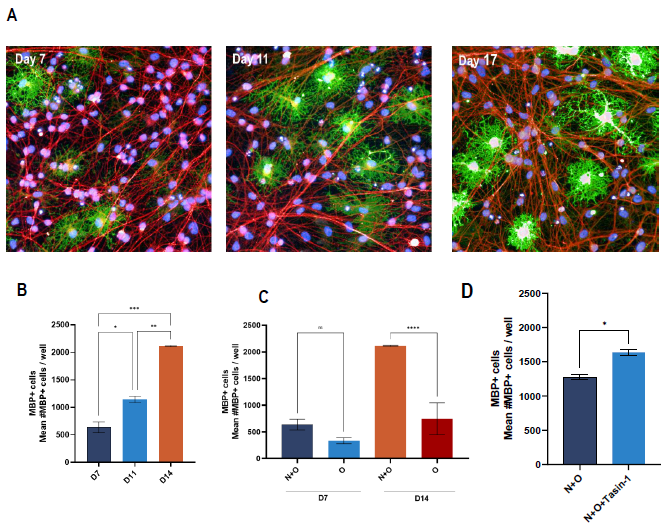
Figure 4. Myelin production in ioGlutamatergic Neuron and ioOligodendrocyte co-culture. (A) Representative images of ioOligodendrocytes and ioGlutamatergic neurons co-culture at different co-culture time points. Cells were stained for DAPI, MBP as oligodendrocyte marker, and beta-III tubulin as neuronal marker. (B) High-content quantification of number of MBP+ cells at different co-culture time points (D) number of MBP+ cells comparing neuronoligodendrocyte co-culture (N+O) versus mono-culture of oligodendrocytes (O) at day 7 and day 14 post-co-culture (N+O) or postthaw (O) and (E) number of MBP+ cells of day 14 N+O co-culture in untreated and Tasin-1 treated cells (C-E) N=2 wells Oneway ANOVA with Tukey’s multiple comparison or unpaired T-test; *p<0.05; **p<0.005; ****p<0.0001; not-significant not indicated]. Image Credit: Charles River Laboratories.
Axonal wrapping in ioOligodendrocyte + ioGlutamatergic neurons co-cultures
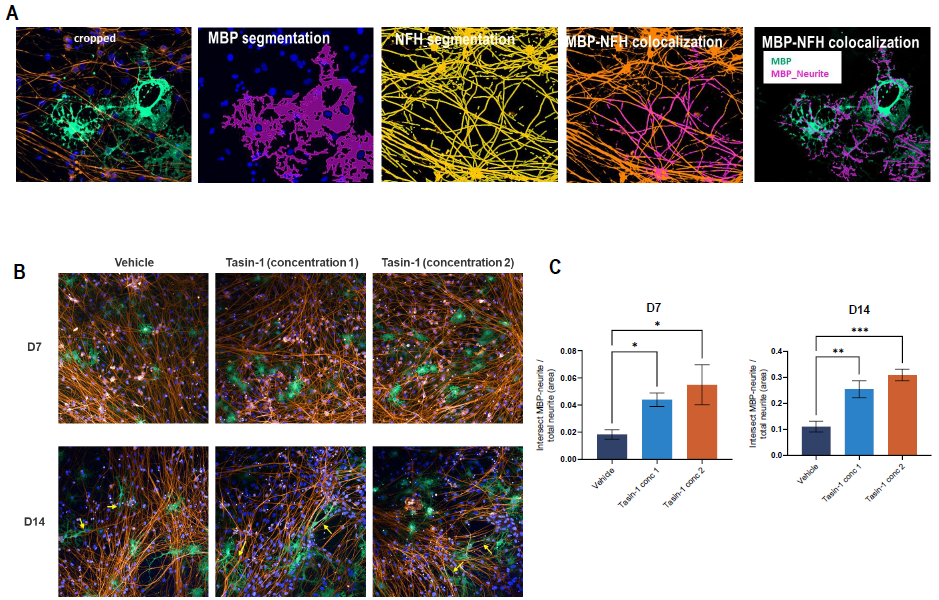
Figure 5. Neurite/axonal wrapping wrapping in ioGlutamatergic Neuron and ioOligodendrocyte co-culture. (A) Segmentation of MBP and Neurofilament H (NFH) signals in a representative image of N+O co-culture. (B) Representative images of day 7 (upper row) and day 14 (lower row) N+O co-cultures stained for DAPI (nuclei), MBP (oligodndrocytes), and NFH (neurites). (C) High-content quantification of the intersection of MBP and NFH signals in day 7 (left graph) and day 14 (right graph) N+O coculture. N=2 wells [One-way ANOVA with Tukey’s multiple comparison; *p<0.05; **p<0.005]. Image Credit: Charles River Laboratories.
Conclusion
Charles River developed a relevant in vitro mono- and co-culture myelination model using iPSC-derived ioOligodendrocyte cells alongside ioGlutamatergic Neurons.
Positive staining for key oligodendrocyte lineage markers was observed via immunofluorescent staining of ioOligodendrocyte cells at different times. This showed Olig2, O4, and SOX2 and myelin markers, including myelin-binding protein (MBP) and myelin proteolipid protein.
At day three after seeding, the O4+ cells exhibited a typical OPC-like morphology in mono-culture, eventually maturing into oligodendrocyte-like cells with typical multiple-branched processes.
A co-culture of ioGlutamatergic Neurons and ioOligodendrocyte cells resulted in more MBP+ cells in a time-dependent fashion versus monocultures of oligodendrocytes.
High content imaging confirmed that MBP+ cells surrounded neurites in the co-culture, demonstrating the successful myelination of neuronal axons.
Acknowledgments
Produced from materials originally authored by Malika Bsibsi, Abeera Popalzij, Matteo Zanella, Lieke Geerts, Mark Musters, Stefan Kostense, and Marijn Vlaming from Charles River, Leiden, Netherlands; David Fischer from Charles River, Chesterford Research Park, UK; and Ludo Buti, Tony Oosterveen, and Ines Ferreira from Bit.Bio, Babraham Research Campus, Cambridge, UK.
About Charles River Laboratories
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work helps to improve the health and well-being of many across the globe.
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies and leading academic institutions around the globe accelerate their research and drug development efforts.
As a fully integrated partner, Charles River can support your research at any point along the drug discovery continuum.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.