This article is based on a poster originally authored by Malika Bsibsi, A. Popalzij, Matteo Zanella, Lieke Geerts, Mark Musters, Inês Ferreira, Stefan Kostense, David F. Fischer and Marijn Vlaming, and presented at SfN 2024.
Neuroinflammation happens in most or all neurodegenerative and inflammatory disorders. In vitro mono-cultures of astrocytes and microglia are effective tools for investigating specific molecular processes implicated in neuroinflammation.
However, more complicated in vitro models are necessary to more accurately model the complexity of the human brain, and to investigate cellular communication in neuroinflammation and neurodegeneration in a human-based model.
The aim of this work is to create a sophisticated in vitro culture model with iPSC-derived neurons (N), astrocytes (A), microglia (M), and oligodendrocytes (O), for use in neuroscience and neurodegeneration drug discovery.
Methods
The various iPSC-derived cell types were cultivated according to manufacturer protocols (ioGlutamatergic neurons, iOligodendrocyte-like cells, ioMicroglia from Bit.bio, and iPSC-derived astrocytes from FujiFilm).
In this model, LPS, nigericin, and beta-amyloid fibrils were used to induce neuroinflammation and neurotoxicity, which were then measured using electrochemiluminescence to determine Neurofilament light chain (NfL), a recognized translational biomarker linked with neurotoxicity and neurodegeneration.
Immunocytochemistry was used to detect DAPI, MBP, beta-III tubulin, and Neurofilament H to test the cultures’ health after treatment. The Yokogawa CV8000 was employed for high-content imaging, and Arivis was used to develop an algorithm for quantifying active nuclei and MBP production.
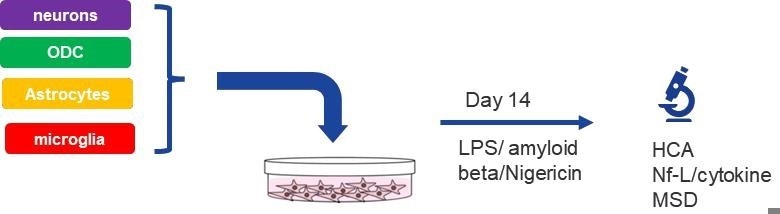
Figure 1. Schematic workflow of multi-cellular co-culture using iPSC-derived CNS cell types. Neurons, astrocytes, oligodendrocytes, and microglia were thawed and seeded at day 0. Treatment with several neuroinflammatory triggers was started at day 14 and performed for 48 hours up to day 16. At day 16, supernatants were harvested, and plates were fixed. Image Credit: Charles River Laboratories.
Results
Phenotypic characterization by immunostaining
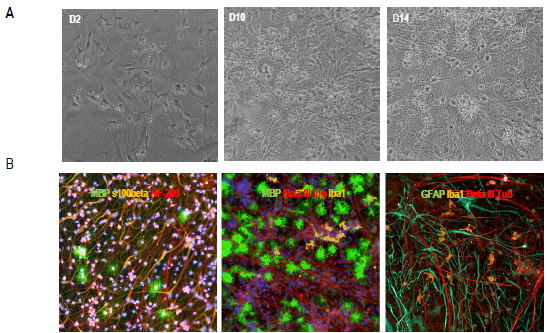
Figure 2. Characterization of the multi-cellular co-culture model. A) Brightfield live images (200x total magnification) at different culture time points post-co-culture. B) Representative images of multicellular co-cultures. Cells were stained for DAPI, MBP as myelin/oligodendrocyte marker, beta-III tubulin or NF-200 to stain neuronal axons, GFAP as astrocyte marker, and Iba1 microglia marker. Image Credit: Charles River Laboratories.
Live nuclei and MBP quantification in multicellular co-cultures after treatment with various stressors
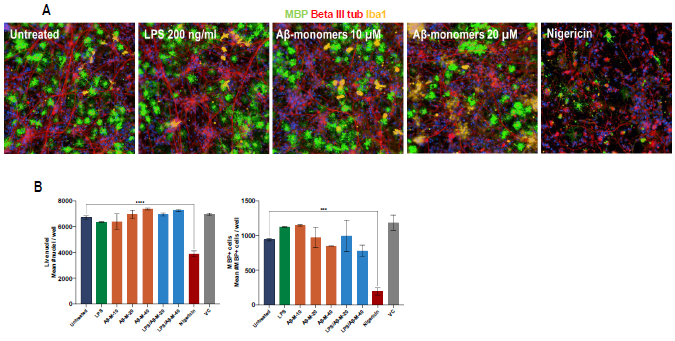
Figure 3. Treatment of iPSC-derived multi-cellular coculture model with neuroinflammatory triggers. A) Representative images of neuron (N), astrocyte (A), oligodendrocyte (O), and microglia co-culture (N+A+O+M) that were (un)treated with 200 ng/ml LPS, 10 μM, 40 μM amyloid-beta aggresure monomers (Aβ-M-40) and 1 μMnigericin. MBP (myelin/oligodendrocyte marker), beta III tubulin (neuronal marker), and Iba1 microglia marker). B) High content quantification of number of live nuclei and MBP+cells of (un)treated N+A+O+M cultures. Image Credit: Charles River Laboratories.
Neurofilament light chain (Nf-L) measurement
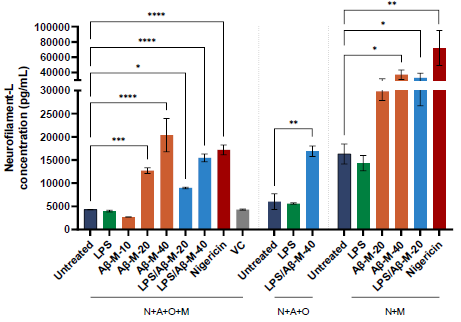
Figure 4. Neurofilament-L (Nf-L) release upon treatment with neuroinflammatory triggers. NF-L release in supernatant harvested after treatment of iPSC-derived multi-cellular co-cultures with different triggers, including LPS and amyloid-beta AggreSure monomers (Aβ-M-40) for 48 hours. Bars represent mean values of duplicate wells with error bars indicating standard deviation. [One-way ANOVA with Tukey’s multiple comparison vs untreated of respective co-culture (N+A+O+M, N+A+O, or N+M) or unpaired T-test; *p<0.05; **p<0.005; ****p<0.0001; not-significant not indicated]. Image Credit: Charles River Laboratories.
Conclusion
Charles River successfully established a multicellular co-culture model combining iPSC-derived glutamatergic neurons, oligodendrocyte-like cells, astrocytes, and microglia, which can be exploited in early-stage drug development.
Triggering acute neuroinflammation resulted in damage to neurons and oligodendrocytes and activation and clustering of microglia, particularly with high concentrations of Aβ monomers. Nigericin caused severe toxicity.
Alongside high imaging, NfL detection in culture supernatants was employed to assess neurotoxicity and degeneration following treatment with various triggers. Neuron-microglia cultures (N+M) showed more NfL release than multicellular cultures N+A+O+M and N+A+O.
About Charles River Laboratories
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work helps to improve the health and well-being of many across the globe.
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies and leading academic institutions around the globe accelerate their research and drug development efforts.
As a fully integrated partner, Charles River can support your research at any point along the drug discovery continuum.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.