Sponsored Content by RefeynReviewed by Olivia FrostJul 18 2024
Mass photometry is a powerful, label-free bioanalytical tool that can evaluate antibody fragmentation and aggregation in response to induced stressors such as pH, light, or peroxide.
Image Credit: ustas7777777
The TwoMP Auto from Refeyn allows users to conduct rapid, automated measurements of multiple samples across a wide mass range whilst ensuring consistently high reproducibility.
Aggregation remains a significant issue for antibodies and can have an adverse effect on both product efficacy and safety.1 A low propensity to form aggregates (from dimers to visible precipitates) is generally desirable throughout manufacturing and product storage.
Forced degradation studies represent a frequently employed approach for the assessment of biopharmaceuticals’ physicochemical stability, including antibody-drug conjugates, monoclonal antibodies, and bispecific antibodies.2
The use of appropriate analytical techniques is essential in such studies if the right candidates are to be selected in the initial stages of the drug development life cycle.
Mass photometry is an ideal tool for gaining rapid insight into changes in molecular behavior, ranging from protein aggregation and degradation to AAV capsid characterization.3 Mass photometry only requires small amounts of sample for analysis, and it is also able to accommodate a diverse array of buffer conditions.
Refeyn’s TwoMP Auto automated mass photometry system is ideal for implementation in wide range of applications, allowing operators to conduct experiments easily thanks to its walkaway automation features.
This article examines the use of the TwoMP Auto during a forced degradation study relating to a panel of therapeutic antibodies. These antibodies were provided by an industrial partner.
Mass photometry characterizes antibody stability in response to stress
The data presented here was part of a wider investigation into the effect of three specific stressors - light, pH, and peroxide - on a panel of 14 different antibodies.
This study was undertaken to ascertain whether exposure to these three stress conditions led to fragmentation and/or aggregation, with each condition measured in triplicate for a total of 168 measurements, including specific control measurements.
The user workflow was simplified through the use of TwoMP Auto’s automation features, with measurements of up to 24 samples per run completed in around 90 minutes. The system was able to do this with only picogram quantities of each antibody.
Data was then analyzed as it was being recorded, with the intuitive software interface allowing the user to begin interpreting results easily and without the need for complicated data analysis routines.
Figures 1 and 2, and Tables 1 and 2, show representative mass photometry data for two of the antibodies from the panel.
When compared to the relevant control sample, it can be observed that each of the antibodies behaved differently in response to the same stress conditions. ‘Antibody 1’ (Figure 1, Table 1) exhibits more pronounced fragmentation, particularly in response to stress condition 2, while ‘Antibody 2’ (Figure 2, Table 2) exhibits a greater degree of aggregation overall.
The results suggest that these antibodies possess very different physiochemical properties while also highlighting the relative abundance of each distinct population of molecules in each antibody sample.
As mass photometry is a single-molecule technique, it has the capacity to detect and count each molecule in a sample. Data acquired, therefore, includes information on a number of rare species, including monomers and dimers.
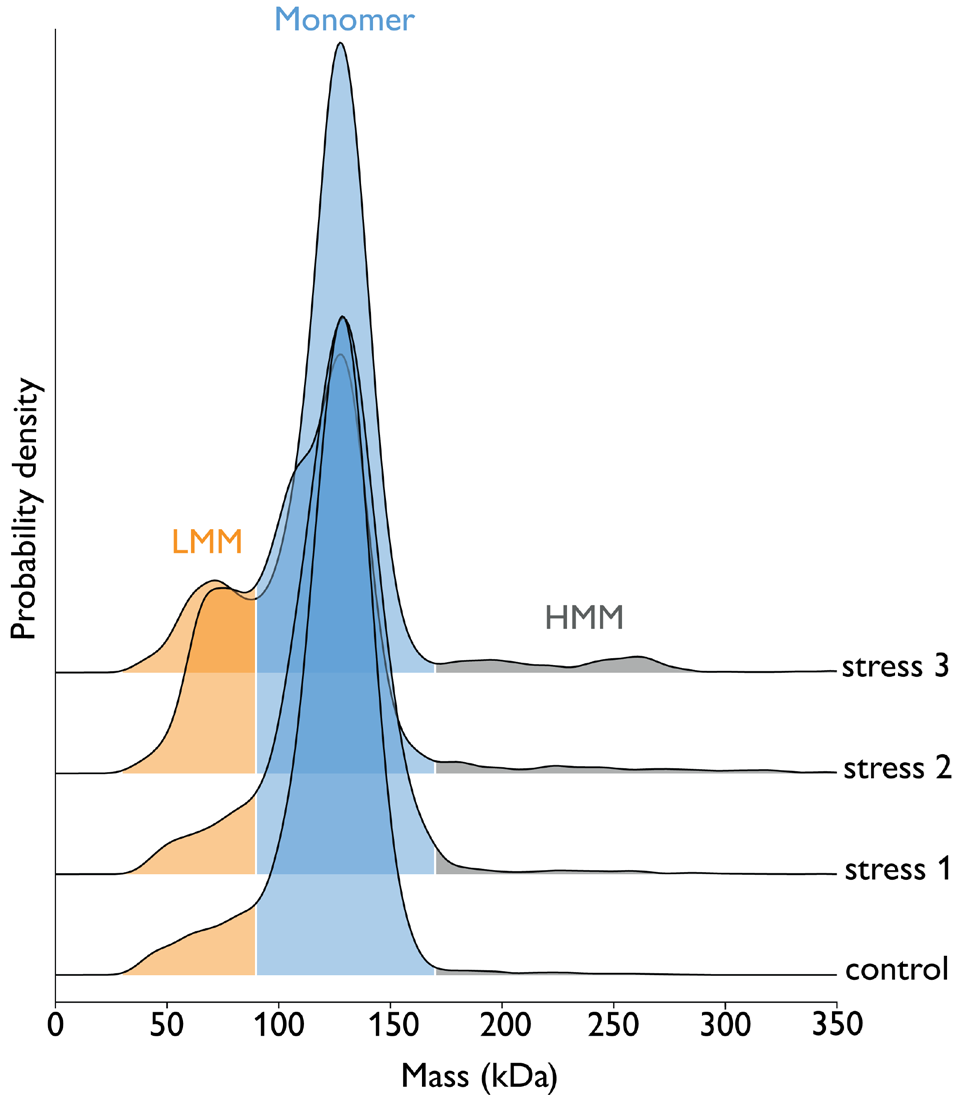
Figure 1. Exposure of ‘Antibody 1’ samples to three different stress conditions. The ‘Stress 1’, ‘2’, and ‘3’ are known to be light, pH change, or peroxide but details of which condition corresponds to which name were not disclosed by the industrial partner. Samples were measured at 10 nM concentration using 5 μl on the TwoMP Auto. Results show that ‘stress 2’ promotes the greatest degree of fragmentation of the antibody, as shown by an increase in the % fraction of ‘low molecular mass’ species (LMM), relative to the control sample. Image Credit: Refeyn Ltd.
Table 1. Fragmentation and aggregation products in Antibody 1 sample upon exposure to different stressors. Low molecular mass fraction (30 - 90 kD) corresponds to [fragmented antibody], monomer mass fraction (90 - 170 kDa) to [intact antibody] and high molecular mass fraction (> 170 kDa) to [aggregated antibody]. Source: Refeyn Ltd.
| Condition |
% Fragmented antibody |
% Intact antibody |
% Aggregated antibody |
| Control |
9% (± 0.7) |
90% (± 0.6) |
1% (± 0.3) |
| Stress 1 |
10% (± 1.1) |
88% (± 1.1) |
2% (± 0.1) |
| Stress 2 |
27% (± 1.8) |
70% (± 1.0) |
4% (± 0.9) |
| Stress 3 |
14% (± 2.2) |
81% (± 2.1) |
5% (± 0.3) |
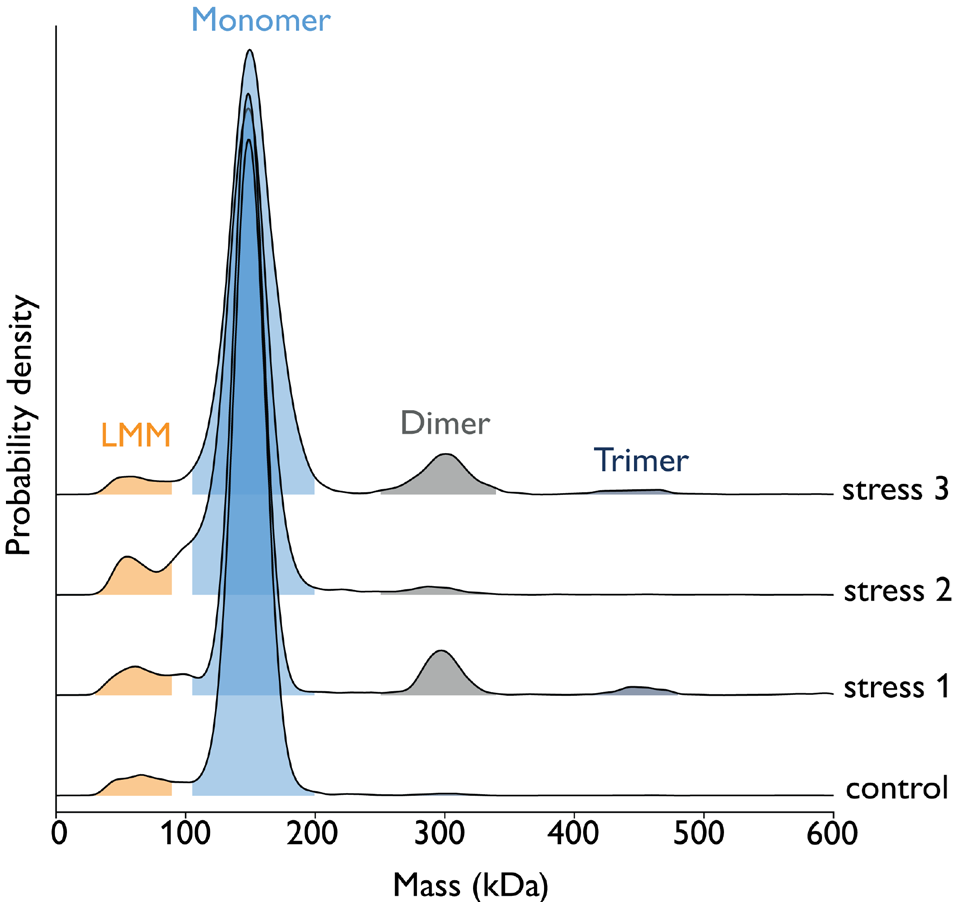
Figure 2. Exposure of ‘Antibody 2’ samples to three different stress conditions (light, pH change, or peroxide). Samples were measured at 10 nM concentration using 5 μl on the TwoMP Auto. Results show that stress conditions 1 and 3 promote aggregation (specifically, the formation of dimers and trimers), relative to the control sample. Image Credit: Refeyn Ltd.
Table 2. Fragmentation and aggregation products in Antibody 2 sample upon exposure to different stressors. Low molecular mass fraction (30 - 90 kDa) corresponds to [fragmented antibody], monomer mass fraction (105 -200 kDa) to [intact antibody], dimer (250 - 340 kDa) and trimer (400 - 480 kDa) mass fractions to [aggregated antibody]. Source: Refeyn Ltd.
| Condition |
% Fragmented antibody |
% Intact antibody |
% Aggregated antibody (dimer) |
% Aggregated antibody (trimer) |
| Control |
4% (± 0.8) |
95% (± 0.7) |
1% (± 0.1) |
0% |
| Stress 1 |
5% (± 0.4) |
85% (± 0.3) |
8% (± 0.4) |
2% (± 0.2) |
| Stress 2 |
7% (± 1.5) |
91% (± 1.4) |
2% (± 0.1) |
0% |
| Stress 3 |
5% (± 0.8) |
86% (± 0.5) |
8% (± 0.3) |
1% (± 0.1) |
Summary
A significant number of degradation products or variants may be generated when forced degradation analyses are performed. Due to this, a number of different analytical tools capable of accommodating a wide mass range should be employed to ensure that all appropriate data is captured.
Mass photometry is especially suited for this application area, representing an ideal complementary approach to techniques such as SEC-MALS.
Mass photometry is also a column-free approach suitable for use with a diverse array range of buffers, resulting in very few sample optimization requirements.
As this study highlights, information about a sample may be captured using only picogram quantities of material. These features are augmented by user-friendly operation and intuitive data analysis, meaning that this technique affords users quick access to valuable new insights.
This data-based decision-making is essential in many areas, such as supporting process development or developability assessments.
Consequently, mass photometry, particularly using the TwoMP Auto, which allows the user to load samples and walk away, can form part of a suite of analytical methods to provide an in-depth characterization of biopharmaceuticals, such as monoclonal and bispecific antibodies.
References and further reading
- Vázquez-Rey & Lang, Biotechnol Bioeng, 2011
- Blessy, Patel et al., JPA, 2014
- Refeyn, How does mass photometry work? [Blog], 2021
Acknowledgments
Produced from materials originally authored by Refeyn Ltd.
About Refeyn Ltd.
Refeyn are the innovators behind mass photometry, a novel biotechnology that allows users to characterise the composition, structure and dynamics of single molecules in their native environment. We are producing a disruptive generation of analytical instruments that open up new possibilities for research into biomolecular functions.
Spun out of Oxford University in 2018 by an experienced team of scientific professionals, Refeyn aims to transform bioanalytics for scientists, academic researchers, and biopharma companies around the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.