NMR spectroscopy can be used to elucidate structures, quantify target compounds and enable synthesis reaction monitoring in real time. It is possible to easily integrate benchtop NMR instruments into pharmaceutical workflows, in applications ranging from manufacturing process and quality control to drug development.
NMR was approved by the US Pharmacopoeia as a primary measurement method because the general quantitative NMR method can provide reliable results without the need for an internal reference standard.
Benchtop NMR instruments such as the X-Pulse benchtop NMR spectrometer are ideally suited to the rapid analysis of synthesized compounds’ composition, purity, and structure. They remove the need to transport samples to off-site NMR facilities.
This instrument offers high-resolution proton NMR, achieving results within just a few minutes and without the need for deuterated solvents.
This article explores the use of Oxford Instruments’ X-Pulse broadband benchtop NMR spectrometer in the structural identification and quantitative analysis of the Active Pharmaceutical Ingredient (API), lansoprazole, as well as the instrument’s wider use cases throughout the field of pharmaceutical research and development.
Benchtop NMR in pharmaceutical workflows
The X-Pulse from Oxford Instruments can facilitate repeatable, cost-effective, and efficient API analysis using 1H NMR. Accurate measurement of APIs is fundamental to ensuring drug efficacy, safety, and quality.
Implementation of NMR can support the research, development, and manufacturing phases, enabling efficient screening, formulation, troubleshooting, and quality control throughout the product development process.
NMR results contain useful information on the amount of substance present in the sample in relation to other components, whether the user is looking to analyze the mixture of components in the sample, or any reference materials used in calibration.

Image Credit: BigKhem/Shutterstock.com
One-dimensional NMR spectroscopy
A significant number of laryngitis patients also suffer from gastroesophageal reflux (GERD). Lansoprazole (Figure 1) is a gastric acid secretion reducer designed to minimize the symptoms of GERD, and an ideal example of an API that can be characterized via 1H NMR spectroscopy.
A one-dimensional NMR measurement of lansoprazole produces a data-rich spectrum, providing useful information on and confirming the API’s structure, composition, and purity.
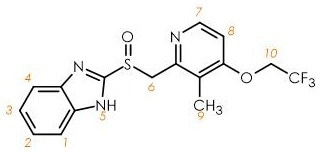
Figure 1. Structure of lansoprazole. Image Credit: Oxford Instruments
Figure 2 features the one-dimensional proton (1H) NMR spectrum for a 209 mmol/ℓ solution of lansoprazole in DMSO-d6. This particular spectrum was collected using a straightforward one-dimensional experiment, whereby a single radiofrequency (RF) pulse is applied to the sample, followed by the acquisition of the NMR signal.
In the example presented here, the upfield resonance of 2.19 ppm is linked to the methyl group 9-CH3, 4.6-5.1 ppm are linked to the 6-CH2 and 10-OCH2 functional groups, 7.0-7.8 ppm are multiplets linked to the aromatic protons, and 8.1-8.4 ppm are linked to the azomethine group.
A broad signal (not shown) was also found at around 13.5 ppm - this is linked to the cyclic amine 5-NH.
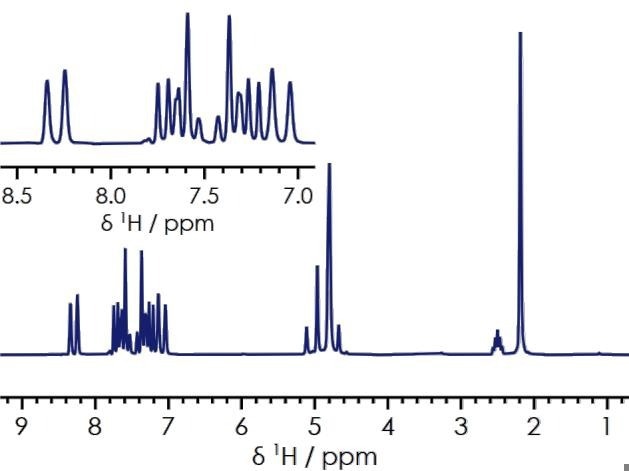
Figure 2. 1H one-dimensional spectrum of lansoprazole in DMSO-d6. Image Credit: Oxford Instruments
This one-dimensional spectrum was acquired in a matter of minutes, and includes enough data to confidently identify the chemical composition of the sample, as well as quantifying the purity of lansoprazole from the various proton environments present.
When appropriately optimized preparation techniques, experimental methods, referencing techniques, and specific acquisition and processing parameters are in place, NMR spectroscopy is an inherently quantitative technique.
In this instance, the quantitative spectrum will directly correlate the observed signal to the relative number of nuclei responsible for generating it.
It is important to note, however, that the precise, accurate determination of analytes is most achievable when the relaxation delay parameter is 5-7 times longer than the T1 for the analyte of interest.
Allowing a long enough relaxation delay for the one-dimensional proton spectrum enables the majority of the system’s nuclear spins to reach bulk magnetization before the RF pulse is applied.
Using the inversion recovery measurement, it is possible to ascertain the required time between radiofrequency (RF) pulses to allow the spins to relax and reach bulk magnetization. The inversion recovery measurement for lansoprazole is displayed in Figure 3.
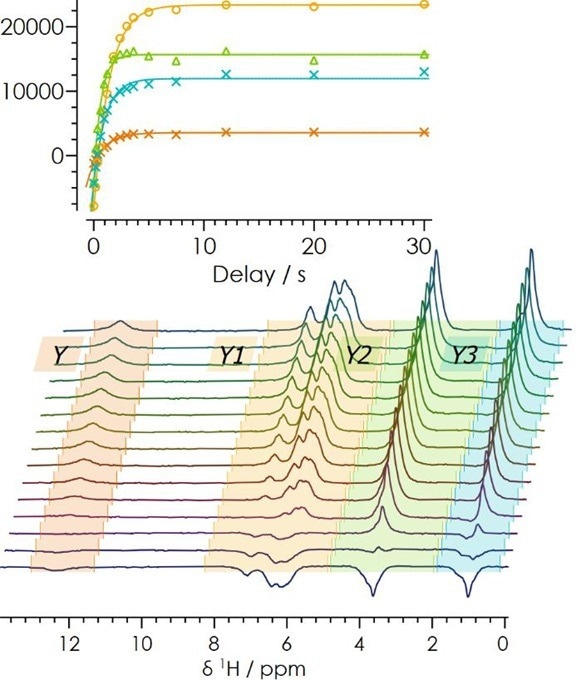
Figure 3. 1H inversion recovery for lansoprazole. Image Credit: Oxford Instruments
By maintaining a relaxation delay of 5-7 times T1, any contributions that may arise from spin-spin and spin-lattice relaxations can be removed. This is a key factor in ensuring accurate quantification.
One-dimensional proton NMR spectra were collected for lansoprazole, and this was done with increasing relaxation delays of 1-20 seconds (Figure 4). It was noted that as the relaxation delay increased, the number of relative nuclei present in solution was more accurately determined.
Once all relevant parameters have been optimized, they can be saved in X-Pulse’s SpinFlow acquisition software, allowing repeat or automated experiments to be performed easily.
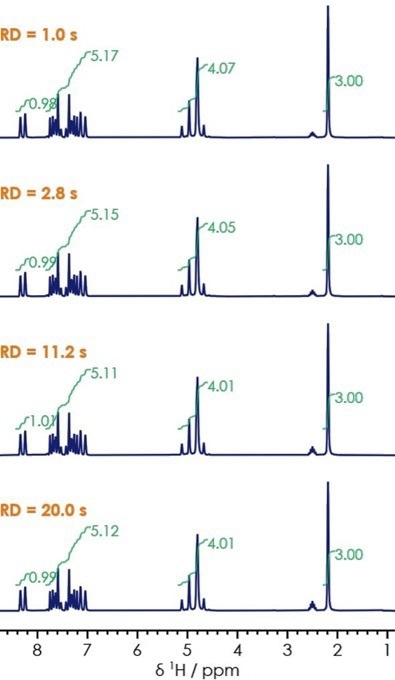
Figure 4. One-dimensional 1H NMR for lansoprazole with varied relaxation delay. Image Credit: Oxford Instruments
Figure 5 shows an acquired 13C NMR spectrum for lansoprazole. Chemical shifts linked to the ester and benzene rings are observed at 161.3, 150.9, and 148.0 ppm, respectively. Alkene signals also appear at 123.1 ppm, nitrogen-substituted rings at 116.1 ppm and 60.0 ppm, and alkanes at 10.43 ppm.
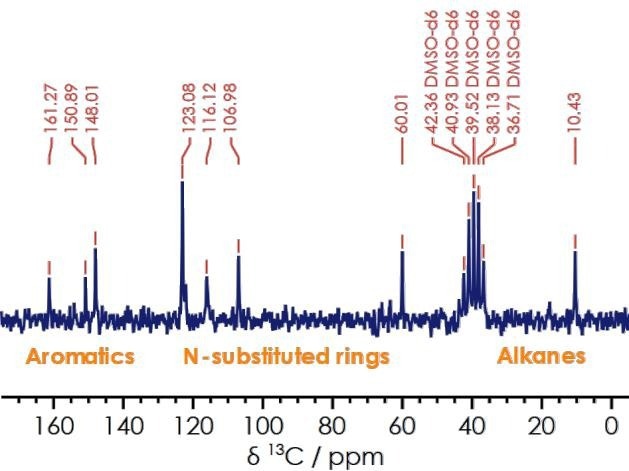
Figure 5. One dimensional 13C{1H} spectrum for lansoprazole. Image Credit: Oxford Instruments
Information acquired via the one-dimensional carbon spectrum enhances users' understanding of the sample’s structure. This is particularly beneficial where a one-dimensional proton NMR features a degree of overlap.
For example, NMR signals linked to the aromatic region in lansoprazole’s proton spectrum are crowded, but carbon-decoupled NMR data enables the user to assign the ester and benzene rings. The decoupled 13C{1H} measurement offers a clearer spectrum without the presence of overlapping peaks.
Two-dimensional NMR spectroscopy
Two-dimensional spectra can complement the analysis of one-dimensional spectra, especially in circumstances where overlapping signals make the one-dimensional spectra too complex to properly interpret.
Where two frequency dimensions result in chemical shift and scalar coupling information, these can be either homonuclear or heteronuclear.
The X-Pulse benchtop NMR spectrometer can provide homonuclear correlative spectroscopy (COSY) measurements within 20 minutes. During the COSY experiment, magnetization is transferred via scalar coupling with protons within 2-3 chemical bonds, yielding cross signals.
The COSY spectrum for lansoprazole in DMSO-d6 is shown in Figure 6. In this example, the diagonal peaks correlate each proton environment with itself while the cross peaks correlate proton-proton couplings.
Clear cross-peak signals can be seen for the azomethine and methyl functional groups (8.32 and 2.19 ppm), methylene and methyl (4.79 and 2.19 ppm), and the azomethine and aromatic protons (7.64 and 8.34 ppm).
Using two-dimensional COSY spectra, it is possible to confidently separate and identify correlations between different protons. This removes any possible signal overlap, which is essential in ensuring accurate structural identification of more complex molecules.
In the example presented here, the COSY spectrum clearly shows the presence of proton J-coupling, a key factor in structural determination.
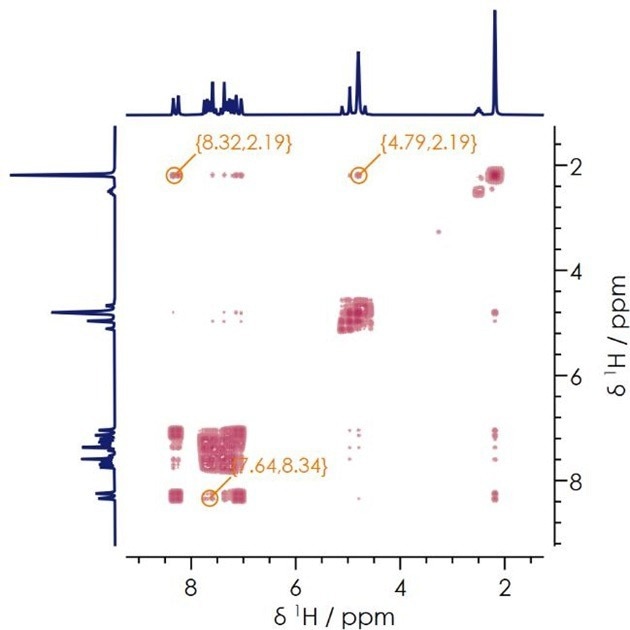
Figure 6. Two dimensional 1H-1H COSY NMR spectrum. Image Credit: Oxford Instruments
The implantation of Heteronuclear Single Quantum Coherence (HSQC) and Heteronuclear Multiple Bond Correlation (HMBC) experiments can provide valuable insight into 1H-13C couplings.
For example, the HSQC experiment outlined here provides single 1H-13C bond coupling information, while the CH and CH3 groups can be distinguished from the CH2 groups by evaluating the signal phase.
The HMBC experiment, meanwhile, highlights connectivity of proton-carbons separated by 2-3 bonds.
Figure 7 features the HSQC and HMBC results for lansoprazole. Within these results, single bond correlations can be seen at δH 2.17 and δC 10.3 ppm (linked to the methyl group, 9-CH3); δH 4.77 and δC 60.0 ppm (linked to the OCH2 and nitrogen substituted ring functional groups); δH 7.02 and δC 107.0 ppm, and δH 7.25, δC 123.1 ppm (linked to the aromatic and nitrogen substituted correlations).
Multiple bond correlations - δH 4.79, δC 54.1 ppm, and δH 8.23, δC 10.41 ppm are linked to the CH2 and nitrogen substituted rings; and azomethine group and alkane correlations, respectively.
The capacity to distinguish between single protons bound to carbons and multiple proton-carbon bond correlations allows users to elucidate the structure of their samples fully.
Two-dimensional spectra become increasingly useful as the complexity of the molecule under investigation increases. The ability to identify the presence or absence of NMR resonances is often vital for structural determination and an essential consideration in pharmaceutical research and development.
While this technique provides instant confirmation that an API is being correctly synthesized in the lab, this is one of many use cases for benchtop NMR instruments.
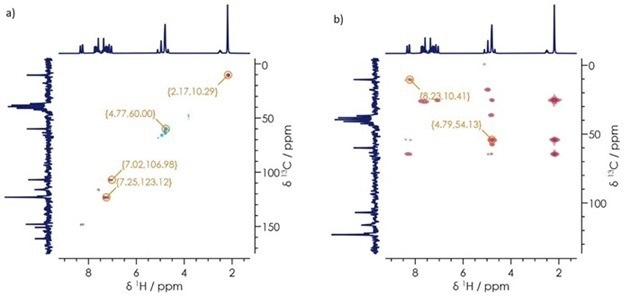
Figure 7. a) 1H-13C HSQC b) 1H-13C HMBC spectra for lansoprazole. Image Credit: Oxford Instruments
The importance of benchtop NMR across pharmaceutical research and development
The case study presented here provided a step-by-step example of investigating the composition, purity, and structure of an API from first principles. In practice, however, benchtop NMR instruments are typically supplied with a range of pre-configured experimental procedures designed to enable the rapid, reliable evaluation of different pharmaceutical materials.
These procedures may include the development of generics, reaction monitoring, screening for purity, fragment screening of small-molecule drug candidates, and metabolomic identification of biomarkers in biofluids.
Pre-configured experiments such as these can facilitate ‘walk-up’ acquisitions that can be employed by virtually any pharmacist, chemist, or technician working in a laboratory environment.
Using an autosampler, an instrument can be operated 24/7 while accommodating much longer duration experiments. Automated analysis routines also enable unattended batch processing of data, which can be employed in real-time adjustment of reaction conditions.
Benchtop NMR instruments are typically compact and moveable, allowing these to be relocated between labs and fume hoods within a lab.
Flow and variable temperature accessories can expand the use of benchtop NMR systems to incorporate dynamic reaction monitoring of synthesis processes, allowing for better assessment of reaction effectiveness or determination of requirements for process scale-up.
Benchtop NMR is inherently quantitative and ideally suited to the identification of both structure and composition. This technique offers distinct advantages over alternative methods such as mass spectroscopy and FTIR spectroscopy.
It is also a non-destructive method, allowing repeated measurements on the same sample without affecting it, and subsequent analysis of the sample using another complementary technique.
Summary
Benchtop NMR is an ideal tool for any pharmaceutical research and development laboratory looking to accurately determine the composition, confirmation, and purity of active pharmaceutical ingredients (API).

Image Credit: Oxford Instruments
The powerful combination of benchtop NMR spectroscopy’s widespread applicability and ease of use, and ongoing advances in automation and flow chemistry, have made NMR a powerful technique suitable for use across several pharmaceutical research and development workflows
These instruments can eliminate the need to transfer samples to central high-field NMR facilities in many cases, enabling instant data collection in virtually any laboratory environment.
All of the experiments outlined in this article were performed using a standard configuration X-Pulse benchtop broadband NMR spectrometer, which can be expanded with an autosampler, flow chemistry, and a range of variable-temperature accessories.
Acknowledgments
Produced from materials originally authored by Oxford Instruments.
About Oxford Instruments
Oxford Instruments is a leading provider of high-technology tools and systems for research and industry, dedicated to accelerating breakthroughs that create a brighter future for our world. With a global presence, we are committed to innovation and excellence, offering cutting-edge solutions that enable researchers and industry professionals to achieve breakthroughs in their fields. Our advanced technologies deliver numerous benefits through unparalleled precision and reliability, allowing users to obtain accurate and reproducible results. By utilising Oxford Instruments' innovative solutions, research is accelerated, productivity is enhanced, and innovation is achieved in various fields, including materials analysis, life sciences, semiconductors, physics, chemistry, and food sciences. We take pride in being a trusted partner for those aiming to push the boundaries of scientific and industrial advancements, providing the tools and support necessary to realise their visions.
Atomic force microscopy: These advanced instruments are utilised for high-resolution imaging and precise measurement of surface properties at the nanoscale level. They offer detailed topographical information and enable accurate measurements of features such as height, roughness, and mechanical properties.
Light microscopy: Our solutions encompass a comprehensive range of advanced imaging systems that utilise visible light for examining samples at the microscale. Equipped with high-quality lenses, cameras, and illumination systems, these optical microscopes deliver detailed images with exceptional clarity and resolution. They find applications in fields such as biology, materials science, and forensics.
Deposition and etching: Our advanced tools are specifically designed for the precise fabrication and modification of materials at the nanoscale. They include a variety of deposition techniques like physical vapor deposition (PVD), chemical vapour deposition (CVD), and atomic layer deposition (ALD), as well as etching processes, such as plasma etching and reactive ion etching (RIE). These tools empower users to create customised materials and devices with exceptional control and precision.
Electron microscopy analysis: Our high-performance tools are tailored for materials characterisation, particle analysis, and sample manipulation at the nanometre scale. Techniques such as Backscatter Electron and X-ray (BEX), Energy Dispersive Spectroscopy (EDS), and Electron Backscatter Diffraction (EBSD) enable imaging, chemical analysis, and crystallographic characterisation of materials at atomic and nanoscale levels.
Optical imaging and spectroscopy: Our optical imaging solutions comprise a range of advanced technologies and systems for capturing and analysing images using light. From state-of-the-art optical microscopes to spectroscopy systems and imaging software, these solutions provide high-quality images and data for applications such as materials characterisation, biological research, and semiconductor analysis.
Nanoindentation: Our high-resolution, MEMS-based nanoindenters are designed to measure the mechanical properties of materials, including hardness, elastic modulus, stiffness, and creep behaviour. These instruments offer superior fabrication tolerances, enhancing sensitivity, resolution, and repeatability beyond conventional technology limits.
Nuclear magnetic resonance: We offer a variety of benchtop Nuclear Magnetic Resonance (NMR) instruments for research, industrial quality assurance/control, and rock core analysis. These instruments provide advanced capabilities for chemical analysis, materials characterisation, and more.
Raman microscopy: Our advanced imaging systems are tailored for high-resolution microscopy and analysis, offering cutting-edge technology and precision optics for detailed insights into samples at the micro- and nanoscales. Trusted by researchers and scientists in fields such as materials science, life sciences, and nanotechnology, these systems are available in various models to suit different applications.
X-ray technologies: Our technologies encompass a range of advanced systems and solutions for materials analysis and characterisation. With cutting-edge capabilities and high sensitivity, Oxford Instruments X-ray technologies are widely used in various industries and research academic institutions for applications such as material identification, quality control, and research in fields like geology, metallurgy, and semiconductor manufacturing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.