NMR spectroscopy is a powerful technique that is ideally suited to the characterization and analysis of a diverse array of chemical compounds.
Unlike many other analytical techniques, NMR spectroscopy can easily distinguish subtle differences in chemical structures.
Isomers are compounds with the same molecular formula but differing structures. They can exhibit a range of chemical and physical properties. A deeper insight into isomerism is key to the development of safer, more effective drugs and their alternatives.
Benchtop NMR spectroscopy can even be used to confidently distinguish between regioisomers, which only differ according to substitution patterns around an aromatic ring.
This article outlines the analysis of several one-dimensional (1H, 13C) and two-dimensional (1H-1H COSY, 1H-13C HSQC) spectra obtained via the Oxford Instruments X-Pulse broadband benchtop NMR spectrometer.
In the examples presented here, differences in spectra - most notably the aromatic regions - are used to distinguish the three regioisomers of hydroxyacetanilide (ortho-, meta-, and para-). These examples showcase the ease with which the regioisomers of small organic molecules can be differentiated.
Para-hydroxy-acetanilide is the most common isomer. The World Health Organization considers this isomer - paracetamol or acetaminophen - the first line of defense against pain.
Paracetamol offers antipyretic (fever prevention and reduction) benefits and can help mitigate allergic symptoms when combined with other drugs.
Image Credit: Olya Abdugalieva/Shutterstock.com
Ortho-hydroxyacetanilide also has an array of everyday uses, such as anti-inflammatory and antirheumatic drugs. Meta-hydroxyacetanilide has not been marketed as a drug thus far, but it does offer some analgesic (pain-relieving) properties.
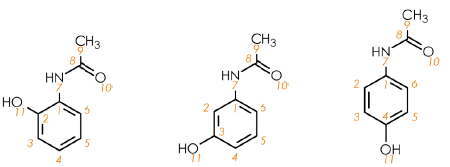
Figure 1. Molecular structures for the hydroxyacetanilide isomers, ortho (left), meta (centre), and para (right). Image Credit: Oxford Instruments
Hydroxyacetanilides
Hydroxyacetanilides are comprised of acetamido {–NHC(O)CH3} and hydroxy (–OH) groups substituted on an aromatic ring. Figure 1 shows the relative substitution positions of two groups around the aromatic ring, as these give rise to three regioisomers: ortho-, meta- and para-.
NMR spectroscopy can distinguish each isomer due to the observable differences in the aromatic regions (δH 6-8 ppm, δC 110-170 ppm) that stem from the various substitution patterns.
A thorough inspection of these samples’ molecular structures enabled the identification of seven chemically unique hydrogen environments and eight chemically unique carbon environments in both the ortho- and meta-isomers.
Symmetry around the aromatic ring in the para-isomer results in just five hydrogen and six carbon environments. This is because in the para-isomer, 2 and 6, and 3 and 5 are chemically equivalent.
These isomers could be distinguished because it is anticipated that each chemically unique environment will give a single signal in an NMR spectrum.
Proton (1H) NMR
Figure 2 displays proton NMR spectra for the three hydroxy-acetanilides assessed. In these three cases, the methyl group 9-CH3 gives a singlet at δH 2 ppm, while broad signals are observed from the hydroxy 11-OH and amine 7-NH hydrogen around δH 9-10 ppm. It is possible to distinguish these compounds by evaluating the remaining aromatic signals in the range δH 6-8 ppm.
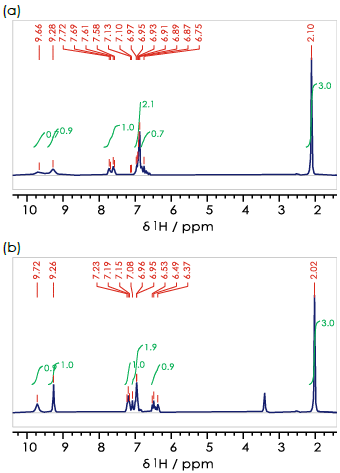
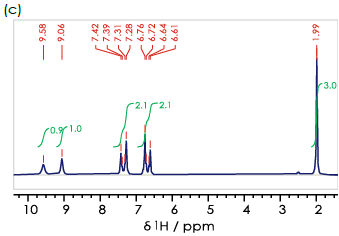
Figure 2. 1H spectra for the (a) ortho- (b) meta- (c) para- isomers of hydroxyacetanilide in DMSO-d6. Image Credit: Oxford Instruments
It is important to note that every chemically unique hydrogen environment will give a single signal in the 1H NMR spectrum.
These signals can be comprised of one or more individual peaks. These signals' number and relative intensities will depend on other nearby hydrogen atoms. It should also be noted that in most instances, ‘nearby’ should be considered to mean separated by at most three chemical bonds, such as H–C–C–H.
In the case of meta-hydroxyacetanilide, 4-, 5- and 6-CH are close enough that each signal will be made up of multiple peaks, while 2-CH is far enough from any other hydrogen atoms that it will yield a signal with a single peak.
Figure 2b confirms that all of these signals are present, but there is a degree of overlap in the aromatic region of the 1H NMR spectrum.
An identical approach can be employed in assessing ortho-hydroxyacetanilide, where it is anticipated that all four aromatic hydrogens would give rise to signals with multiple peaks that exhibit a degree of overlap at benchtop frequencies.
Para-hydroxyacetanilide is different, however. Its molecular symmetry results in two observable signals, including two main peaks arising from a single three-bond interaction (for example, 2-H–C–C–H-3). This noteworthy difference means that para-hydroxyacetanilide (paracetamol, Figure 2c) can be distinguished from the other regioisomers investigated.
The notable signal overlap in this case means that more information would be required to assign all three regioisomers unequivocally via NMR spectroscopy. A simple one-dimensional proton spectrum would not be sufficient in this instance.
Carbon-13 (13C) NMR
One means of addressing this issue is to evaluate one-dimensional carbon-13 spectra. Because these spectra are acquired proton-decoupled, each chemical environment gives rise to a single peak in the NMR spectrum (Figure 3).
The methyl group 9-CH3 gives a signal at δC 23 ppm for all three compounds, while 8-CO gives a peak at δC 168 ppm, and aromatic carbons 1-6 give signals over a range of 160-105 ppm.
Para-hydroxyacetanilide can easily be identified via its proton NMR spectrum because its symmetry means there are just four unique chemical environments in the the aromatic ring (1-C, 2/6-CH, 3/5-CH, 4-C), and therefore only four aromatic signals are present in the 13C{1H} NMR spectrum (Figure 3c).
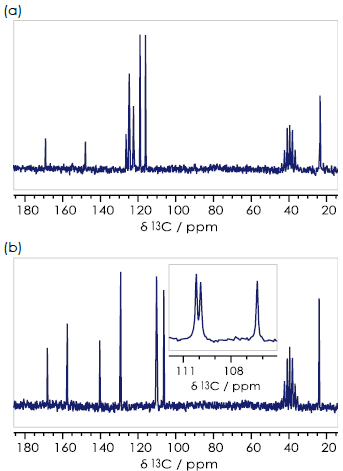
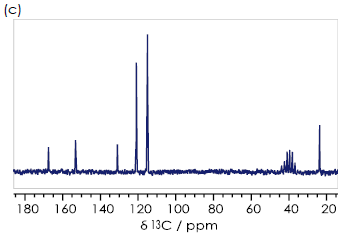
Figure 3. 13C {1H} spectra for the (a) ortho- (b) meta- (c) para- isomers of hydroxyacetanilide in DMSO-d6. Image Credit: Oxford Instruments
Ortho- (Figure 3a) and meta-hydroxyacetanilide (Figure 3b) have six aromatic signals. It may be feasible to assign each of these signals to the appropriate aromatic carbon by employing a theoretical understanding of the origin of chemical shifts.
Two-dimensional spectra
One-dimensional 1H and 13C NMR spectra can provide a good amount of structural information, but it is possible to combine two-dimensional NMR spectra - specifically, the signal dispersion of the 13C spectra and the informational content of the 1H spectra can be combined - to provide even more insight into the sample’s structure.
This combination of two-dimensional NMR spectra allows users to unequivocally distinguish the three regioisomers.
1H-13C correlation spectra
A two-dimensional 1H-13C HSQC (Heteronuclear Single Quantum Coherence) spectrum displays one-dimensional 1H and 13C spectra along the x- and y-axes, respectively.
Cross peaks in this spectrum correspond to one bond 1H-13C interactions, meaning it is possible to connect 13C environments to their corresponding 1H environments.
The 1H-13C HSQC spectra features aromatic regions for the three hydroxyacetanilide regioisomers (Figure 4). In every case, each signal is clearly resolved by showing the data in two-dimensions. This allows all three regioisomers to be identified and distinguished.
In the example presented here, the most easily identifiable isomer continues to be para-hydroxyacetanilide (Figure 4c). The two signals here are distinguished, appearing as two distinct peaks.
In the case of the ortho- (Figure 4a) and meta- (Figure 4b) isomer, it is possible to observe four signals in the two-dimensional HSQC spectrum. A signal comprising a single sharp peak can be identified as meta-hydroxyacetanilide (δH + 6.96, δC +109.9 ppm), which corresponds to 2-CH.
Interactions between adjacent CH groups in the molecules result in all other signals in the HSQC exhibiting clear broadening (or multiple peaks) in the 1H dimension.
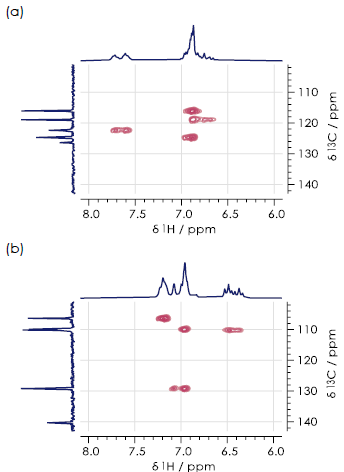
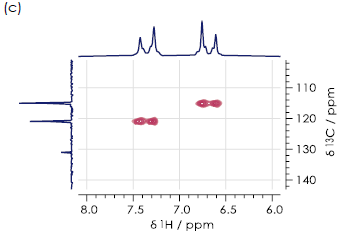
Figure 4. Aromatic regions of 1H-13C HSQC spectra for the (a) ortho- (b) meta- (c) paraisomers of hydroxyacetanilide in DMSO-d6. Image Credit: Oxford Instruments
1H-1H correlation spectra
A 1H-1H COSY (Correlation Spectroscopy) spectrum represents another type of two-dimensional NMR spectrum ideally suited to distinguishing regioisomers. This technique is useful where off-diagonal signals appear in the spectrum due to coupling between 1H NMR signals. This effect gives rise to signals exhibiting multiple peaks in the one-dimensional spectrum.
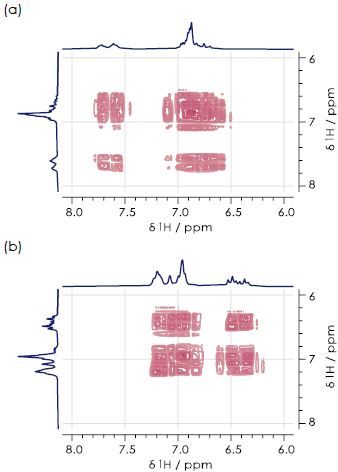
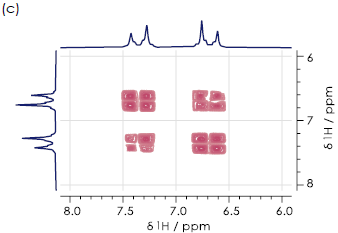
Figure 5. Aromatic regions of 1H-1H COSY spectra for the (a) ortho- (b) meta- (c) paraisomers of hydroxyacetanilide in DMSO-d6. Image Credit: Oxford Instruments
Figure 5 displays aromatic regions of the 1H-1H COSY spectra for all three hydroxyacetanilide regioisomers. It was not possible to distinguish these in this case, due to the presence of substantial signal overlap.
Summary
This article investigated the chemical structures of three regioisomers: ortho-, meta- and para-hydroxyacetanilide. Their structures were explored using a combination of one- and two-dimensional NMR spectra, all acquired using an Oxford Instruments X-Pulse broadband benchtop NMR spectrometer.

Image Credit: Oxford Instruments
These spectra facilitate the clear differentiation between three isomers of the same small organic molecule hydroxyacetanilide. These isomers are key to the pain- and fever-relieving characteristics of paracetamol.
The X-Pulse broadband benchtop NMR spectrometer can be provided with a comprehensive range of standardized one- and two- dimensional characterization sequences and analyses.
An optional 25-position autosampler is also available, allowing users to maximize throughput and efficiency, for example, when identifying pharmaceutically relevant target molecules via screening studies.
Acknowledgments
Produced from materials originally authored by Oxford Instruments.
About Oxford Instruments
Oxford Instruments is a leading provider of high-technology tools and systems for research and industry, dedicated to accelerating breakthroughs that create a brighter future for our world. With a global presence, we are committed to innovation and excellence, offering cutting-edge solutions that enable researchers and industry professionals to achieve breakthroughs in their fields. Our advanced technologies deliver numerous benefits through unparalleled precision and reliability, allowing users to obtain accurate and reproducible results. By utilising Oxford Instruments' innovative solutions, research is accelerated, productivity is enhanced, and innovation is achieved in various fields, including materials analysis, life sciences, semiconductors, physics, chemistry, and food sciences. We take pride in being a trusted partner for those aiming to push the boundaries of scientific and industrial advancements, providing the tools and support necessary to realise their visions.
Atomic force microscopy: These advanced instruments are utilised for high-resolution imaging and precise measurement of surface properties at the nanoscale level. They offer detailed topographical information and enable accurate measurements of features such as height, roughness, and mechanical properties.
Light microscopy: Our solutions encompass a comprehensive range of advanced imaging systems that utilise visible light for examining samples at the microscale. Equipped with high-quality lenses, cameras, and illumination systems, these optical microscopes deliver detailed images with exceptional clarity and resolution. They find applications in fields such as biology, materials science, and forensics.
Deposition and etching: Our advanced tools are specifically designed for the precise fabrication and modification of materials at the nanoscale. They include a variety of deposition techniques like physical vapor deposition (PVD), chemical vapour deposition (CVD), and atomic layer deposition (ALD), as well as etching processes, such as plasma etching and reactive ion etching (RIE). These tools empower users to create customised materials and devices with exceptional control and precision.
Electron microscopy analysis: Our high-performance tools are tailored for materials characterisation, particle analysis, and sample manipulation at the nanometre scale. Techniques such as Backscatter Electron and X-ray (BEX), Energy Dispersive Spectroscopy (EDS), and Electron Backscatter Diffraction (EBSD) enable imaging, chemical analysis, and crystallographic characterisation of materials at atomic and nanoscale levels.
Optical imaging and spectroscopy: Our optical imaging solutions comprise a range of advanced technologies and systems for capturing and analysing images using light. From state-of-the-art optical microscopes to spectroscopy systems and imaging software, these solutions provide high-quality images and data for applications such as materials characterisation, biological research, and semiconductor analysis.
Nanoindentation: Our high-resolution, MEMS-based nanoindenters are designed to measure the mechanical properties of materials, including hardness, elastic modulus, stiffness, and creep behaviour. These instruments offer superior fabrication tolerances, enhancing sensitivity, resolution, and repeatability beyond conventional technology limits.
Nuclear magnetic resonance: We offer a variety of benchtop Nuclear Magnetic Resonance (NMR) instruments for research, industrial quality assurance/control, and rock core analysis. These instruments provide advanced capabilities for chemical analysis, materials characterisation, and more.
Raman microscopy: Our advanced imaging systems are tailored for high-resolution microscopy and analysis, offering cutting-edge technology and precision optics for detailed insights into samples at the micro- and nanoscales. Trusted by researchers and scientists in fields such as materials science, life sciences, and nanotechnology, these systems are available in various models to suit different applications.
X-ray technologies: Our technologies encompass a range of advanced systems and solutions for materials analysis and characterisation. With cutting-edge capabilities and high sensitivity, Oxford Instruments X-ray technologies are widely used in various industries and research academic institutions for applications such as material identification, quality control, and research in fields like geology, metallurgy, and semiconductor manufacturing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.