In drug development and discovery, NMR spectrometry plays a pivotal role in the analysis of molecular structures. NMR screening methods provide a valuable and reliable tool for hit-to-lead optimization and the identification of small molecules.
Usually, a compound library is checked for “hits” (such as particular ligands) that can bind to a specific target. The hits can then be validated and advanced for potential future development using NMR binding tests. However, this application is typically limited to higher field NMR instruments.
Benchtop NMR excels in the later stages of the drug development and manufacturing process, where the accurate identification of small molecules continues to be a critical factor. The requirements of an approved “reference standard” must be met by all drug products. It is common for NMR to provide the information necessary to create these standards and also be applied in-process to make sure that the intermediates and end products consistently meet them.
In several cases, a simple one-dimensional hydrogen spectrum can quickly verify a structure based on the peak splitting, chemical shift, and integral value. For simple small molecules, a significant portion of this analysis can be automated using standard routines.
If the molecule is more complex, some signals in the one-dimensional spectrum may overlap. A high-performance benchtop NMR spectrometer, like the X-Pulse, can provide a variety of one-dimensional and two-dimensional experiments to allow structural confirmation and even whole-molecule structural elucidation for unknowns.
Structure of the drug gemfibrozil
Figure 1 illustrates the chemical composition of the drug gemfibrozil, also known by its IUPAC name 5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoic acid and its empirical formula C15H22O3. As a member of the fibrate class of molecules, it regulates blood lipids by reducing triglyceride and LDL cholesterol levels while raising HDL levels, thereby reducing the risk of heart disease.
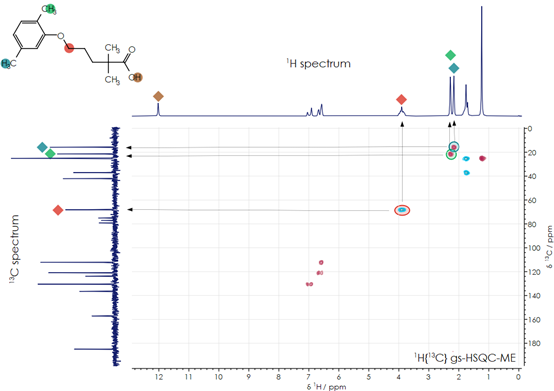
Figure 1. Gemfibrozil 1D and 2D NMR spectra. Image Credit: Oxford Instruments
Figure 1 depicts the fully decoupled carbon spectrum (left), the one-dimensional hydrogen spectrum (top), and the two-dimensional 1H–13C HSQC spectrum. In the HSQC spectrum, plotted as a contour map, peaks can be observed at the chemical shift coordinates corresponding to the shift of a hydrogen nucleus and the relative carbon nucleus to which it is bonded. The arrows in the diagram illustrate these examples.
A 1H-13C HMBC spectrum can be employed to assign longer-range through-bond correlations between hydrogen and carbon nuclei within the molecule. By mixing data obtained from these spectra, the characteristic peaks of the molecule can be identified clearly, and the molecular structure of the active end product can be confirmed.

 Download application note for extended discussion on structural characterisation by NMR looking at Ibuprofen
Download application note for extended discussion on structural characterisation by NMR looking at Ibuprofen
About Oxford Instruments
Oxford Instruments is a leading provider of high-technology tools and systems for research and industry, dedicated to accelerating breakthroughs that create a brighter future for our world. With a global presence, we are committed to innovation and excellence, offering cutting-edge solutions that enable researchers and industry professionals to achieve breakthroughs in their fields. Our advanced technologies deliver numerous benefits through unparalleled precision and reliability, allowing users to obtain accurate and reproducible results. By utilising Oxford Instruments' innovative solutions, research is accelerated, productivity is enhanced, and innovation is achieved in various fields, including materials analysis, life sciences, semiconductors, physics, chemistry, and food sciences. We take pride in being a trusted partner for those aiming to push the boundaries of scientific and industrial advancements, providing the tools and support necessary to realise their visions.
Atomic force microscopy: These advanced instruments are utilised for high-resolution imaging and precise measurement of surface properties at the nanoscale level. They offer detailed topographical information and enable accurate measurements of features such as height, roughness, and mechanical properties.
Light microscopy: Our solutions encompass a comprehensive range of advanced imaging systems that utilise visible light for examining samples at the microscale. Equipped with high-quality lenses, cameras, and illumination systems, these optical microscopes deliver detailed images with exceptional clarity and resolution. They find applications in fields such as biology, materials science, and forensics.
Deposition and etching: Our advanced tools are specifically designed for the precise fabrication and modification of materials at the nanoscale. They include a variety of deposition techniques like physical vapor deposition (PVD), chemical vapour deposition (CVD), and atomic layer deposition (ALD), as well as etching processes, such as plasma etching and reactive ion etching (RIE). These tools empower users to create customised materials and devices with exceptional control and precision.
Electron microscopy analysis: Our high-performance tools are tailored for materials characterisation, particle analysis, and sample manipulation at the nanometre scale. Techniques such as Backscatter Electron and X-ray (BEX), Energy Dispersive Spectroscopy (EDS), and Electron Backscatter Diffraction (EBSD) enable imaging, chemical analysis, and crystallographic characterisation of materials at atomic and nanoscale levels.
Optical imaging and spectroscopy: Our optical imaging solutions comprise a range of advanced technologies and systems for capturing and analysing images using light. From state-of-the-art optical microscopes to spectroscopy systems and imaging software, these solutions provide high-quality images and data for applications such as materials characterisation, biological research, and semiconductor analysis.
Nanoindentation: Our high-resolution, MEMS-based nanoindenters are designed to measure the mechanical properties of materials, including hardness, elastic modulus, stiffness, and creep behaviour. These instruments offer superior fabrication tolerances, enhancing sensitivity, resolution, and repeatability beyond conventional technology limits.
Nuclear magnetic resonance: We offer a variety of benchtop Nuclear Magnetic Resonance (NMR) instruments for research, industrial quality assurance/control, and rock core analysis. These instruments provide advanced capabilities for chemical analysis, materials characterisation, and more.
Raman microscopy: Our advanced imaging systems are tailored for high-resolution microscopy and analysis, offering cutting-edge technology and precision optics for detailed insights into samples at the micro- and nanoscales. Trusted by researchers and scientists in fields such as materials science, life sciences, and nanotechnology, these systems are available in various models to suit different applications.
X-ray technologies: Our technologies encompass a range of advanced systems and solutions for materials analysis and characterisation. With cutting-edge capabilities and high sensitivity, Oxford Instruments X-ray technologies are widely used in various industries and research academic institutions for applications such as material identification, quality control, and research in fields like geology, metallurgy, and semiconductor manufacturing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.