May 31 2018
Nanoparticles with multifunctional drug precursor for synergistic tumor therapy
For efficient cancer therapy with few side effects, the active drug should selectively attain high concentration in the tumor. In the journal Angewandte Chemie, scientists have introduced a new approach, in which two synergistic drug components are combined into a dimer. This dimer can be incorporated into polymeric nanotransporters at exceptionally high concentration. The components are activated when the dimer is split within the tumor. In addition, they enable use of two different imaging techniques.
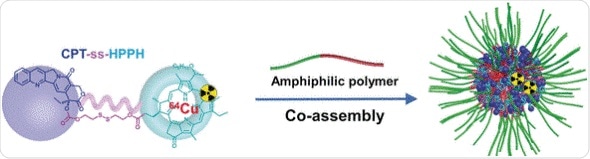
Polymeric micelles are the most important nanotransporters used in treating tumors. Despite improved transport systems, many challenges must still be overcome: insufficient loading, premature release of the drug, no ability to monitor distribution of the drug, and limited accumulation of the drug within the tumor tissue. Longjiang Zhang, Guizhi Zhu, Xiaoyuan Chen, and their team have approached these problems from the other direction. Instead of improving the transporter, they improved the cargo.
The scientists from the National Institutes of Health in Bethesda, USA, and Nanjing University, China, have used a simple but effective trick: they connected two drugs, camptothecin and a special photosensitizer, to make a dimer. Micelles can very efficiently be loaded with an unusually large amount of the dimeric freight (59%). The dimers are less hydrophilic than their individual components, allowing them to be more easily introduced into the hydrophobic interior of the micelles. For the same reason, the dimers do not exit the micelles as they travel through the blood vessels. This reduces undesirable side effects.
Both of the components of the initially inactive dimer are connected by a disulfide bridge that can only be broken by a glutathione-dependent reaction cascade. Glutathione is a small protein that is present in high concentration in many tumors. Both of the drugs are only activated after the dimer is split within the tumor cells.
When the area of the tumor is irradiated with laser light, the photosensitizer converts normal oxygen into highly reactive singlet oxygen, which damages the cell and causes an oxygen deficiency. Camptothecin inhibits factor 1α, which helps cells to withstand oxygen deficiency. This boosts the cytotoxic effect of the photosensitizer. Another effect of camptothecin is that it damages the tumor cells’ DNA.
In addition, the photosensitizer is a fluorescent dye and it can bind the radioisotope copper-64, which enables visualization with both fluorescence imaging and positron emission tomography (PET). Quantitative PET allows for precise monitoring of the dimer, as well as confirmation of its pharmacokinetics and biodistribution in vivo.
Experiments with cell cultures and tumorous mice demonstrated that this new method significantly improved transport and accumulation of the drug in tumors with significantly fewer side effects, while shrinking the tumor to a significantly greater degree than administration of the unbound individual components.
Source: http://newsroom.wiley.com/press-release/angewandte-chemie-international-edition/combination-pack-battles-cancer-nanoparticles-