Sponsored Content by PittconJun 14 2019
An interview with Renee Frontiera from the University of Minnesota, discussing the development of a super-resolution Raman microscope that could one day supersede fluorescence-based super-resolution microscopes.
What are the main limitations of super-resolution microscopy?
Most far-field super-resolution microscopies use fluorescence labeling. A major problem with this is that it requires fluorophores, which often bleach during the timescale of the experiment. This means the signal will turn off in the middle of a measurement, and no further analysis can be carried out. Therefore, being able to do super-resolution without bleaching or large chromophores that could perturb dynamics, could be fantastic.
 Micha Weber | Shutterstock
Micha Weber | Shutterstock
How can Raman spectroscopy be used to overcome these issues?
Raman spectroscopy is intrinsically label-free. You can use it with very small two atom labels to provide more sensitivity but in most cases, you do not need to go in and label your sample. Instead, you can look at several chemical components simultaneously through their unique Raman spectra.
As Raman spectroscopy doesn’t involve the use of chromophores, there is no bleaching. This resolves the issue of bleaching that is commonly seen in super-resolution microscopy.
Please describe the super-resolution Raman microscopy technique that you recently developed.
Our technique combines two different techniques that are already available. From the microscopy community, we borrowed ideas from Stimulated Emission Depletion Microscopy (STED). We combined this with expertise from the field of Stimulated Raman Microscopy to create a super-resolution microscopy technique.
Essentially, our microscope involves a three-laser beam technique, where we generate a stimulated Raman signal in a sample, and then we can turn it off in a donut-shaped region around this initial excitation spot. We get a Raman signal only from the center of this donut. This is similar to how STED works with fluorescence.
What are the main advantages of this technique?
The main advantage of this system is that it is label-free, so you do not need to do any sample prep beforehand to target different chemical species in your system.
Another key advantage is that you can look at several components simultaneously. For example, in a cell, we can see proteins, lipid species, and small molecules that we add to the system. We can track all of these in real space and real time, without the need for any extensive sample prep or labeling. It is highly advantageous in that sense.
There are also a number of systems outside of biology where labeling is just not possible. For example, take organic photovoltaics or electrochemical systems. It is very hard, if not impossible, to integrate fluorescence labels into those systems. An atmospheric optical imaging technique to look at these on nanometer length scales opens up new opportunities.
Ultrafast and Nanoscale
Ultrafast and Nanoscale from AZoNetwork on Vimeo.
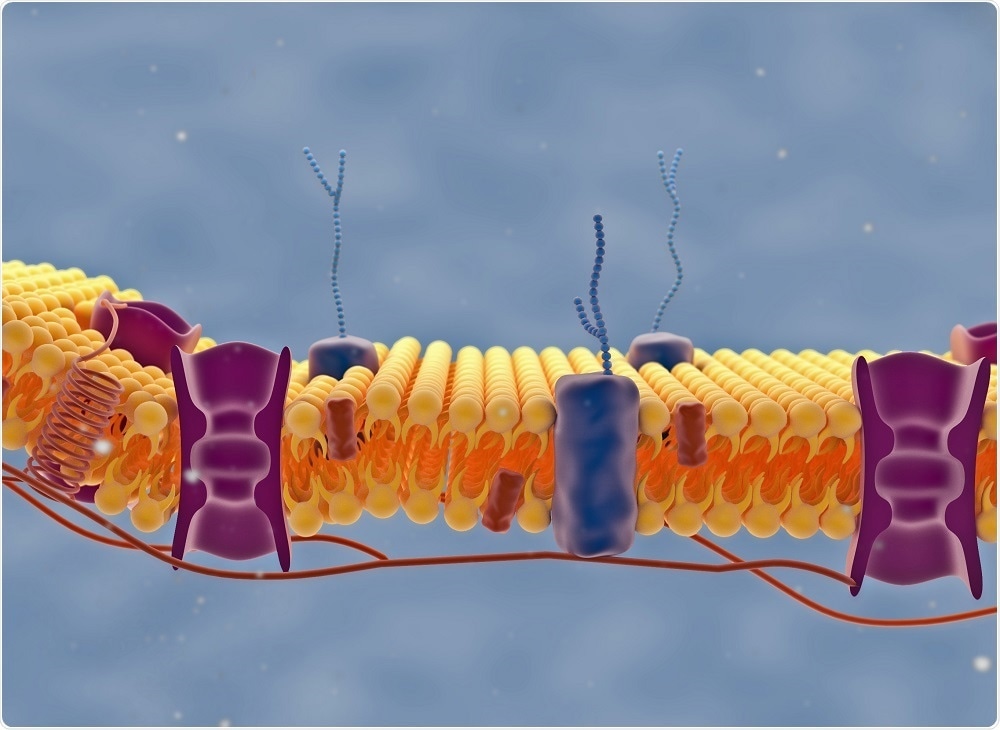
The new technique breaks the optical diffraction limit. Why is this useful for studying cellular lipid dynamics?
If you look at a cell membrane, you will see ~400,000 lipid molecules. We know that cell membranes are very heterogeneous on nanometer length scales and that they vary in composition, thickness, fluidity and curvature on these very short length scales. However, we have no idea what that means for their function because it is so hard to look at.
Breaking this optical diffraction limit means that we can probe regions around single proteins, instead of averaging over all these regions. We can also figure out how these local structures impact function.
It is important for R&D scientists to understand how a new compound interacts with cells. How can this technique be used to understand drug delivery mechanisms?
At present, around 70% of pharmaceuticals target trans-membrane proteins. We have no idea how the local environment around these proteins affects the ability of the drug molecule to bind and then impact signal transduction. What we would really like to know is what the structure-function relationships are between the membrane environment and the pharmaceutical performance. Our technique allows us to do this, by focusing in on specific components of the cell membrane.
 Marco G Faria | Shutterstock
Marco G Faria | Shutterstock
What are plasmonic nanomaterials and how can plasmonic substrates improve Raman spectroscopy?
Plasmonic materials are remarkable in how they interact with light. If you shine light on plasmonic materials, they can concentrate that light down to nanometer length scales. They can also generate hotspot regions of enhanced electromagnetic field intensity.
If you stick a molecule in one of those hotspot regions, you see dramatically increased Raman signals to the point that you can do single molecule surface enhanced Raman spectroscopy. You boost the signal by many orders of magnitude in these very localized regions.
Understanding chemical interactions is a key part of drug development. How can plasmon-enhanced Raman techniques be used to study reactions in real-time, at ultrafast speeds?
There is a lot of excitement in the plasmonics world right now regarding doing chemistry in these hotspot regions. If you stick a molecule in this region of very enhanced electromagnetic field intensity, there is a lot of heat and a lot of highly energetic electrons available. This led to the question, what sort of chemical reactions could you do in that very unique environments?
People have shown that you can do catalysis with sunlight and these nanomaterials. There is not a lot known about the mechanism. What we are doing with ultra-fast Surface Enhanced Raman techniques (SERs), is trying to understand how these plasmons interact with molecules, how they break and make bonds, and trying to watch these processes in real time, to understand the mechanism behind this catalysis.
 Juan Gaertner | Shutterstock
Juan Gaertner | Shutterstock
A major limitation of this technique is that plasmons can damage samples and interfere with research results. Can this be avoided or reduced?
There have been a lot of questions about heat-related damage. That is, how much are these plasmonic materials heating up samples or damaging samples? In some cases, this is really advantageous. There is a whole class of plasmonics research on photothermal therapy. If you can target these materials to say cancer cells and heat them up, they damage the materials, which is exactly what you want.
However, in cases like the catalysis field, heating and associated damage is not particularly beneficial. This is a very expensive way to heat up molecules. We have much cheaper and more efficient ways of doing this.
One of the things we have been doing in my group is trying to understand how the plasmons transfer energy to molecules in ways that might lead to heat associated damage. We are trying to see if we can tune the plasmonic material or the surrounding environment to try and control that on these very fast timescales.
What do the next few years look like for your research team?
We are excited about applying some of the Raman techniques that we have been developing to newly identified problems. For example, photocatalysis and solar energy conversion, and designing materials or optical cavities to promote certain reactions.
I think there are a lot of exciting applications for this super-resolution Raman technique where it can provide information that existing technologies cannot. For example, in human health, energy conversion, or in battery research. We are excited about collaborating and trying a lot of these different areas.
What have you enjoyed about Pittcon 2019? Would you recommend the conference to others in your field?
Pittcon is amazing in terms of the breadth of analytical chemistry research. I would say that my group does not define ourselves solely as analytical chemists. Hence, being able to get different perspective on different fields of analytical chemistry is phenomenal.
The scale of the conference and the number of concurrent talks are going on amazes me. It is a wonderful place to talk to and meet people in spectroscopy, electrochemistry, mass spectrometry and many other analytical areas.
Where can readers find more information?
About Renee Frontiera
 Renee Frontiera is an Associate Professor and McKnight Land-Grant Professor in the Department of Chemistry at the University of Minnesota. She received her Ph.D. in 2009 from the University of California, Berkely, and moved on to a Post Doctorate position at North Western University. She has been working at the University of Minnesota since 2013.
Renee Frontiera is an Associate Professor and McKnight Land-Grant Professor in the Department of Chemistry at the University of Minnesota. She received her Ph.D. in 2009 from the University of California, Berkely, and moved on to a Post Doctorate position at North Western University. She has been working at the University of Minnesota since 2013.
Frontiera's research group investigates fundamental and applied issues in membrane protein biophysics, alternative energy sources, and nanotechnology.
The group aims to develop and apply new spectroscopic and microscopic techniques to examine how molecules react, following their dynamics on the nanometer length scale with femtosecond time resolution.
About Pittcon
 Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Proceeds from Pittcon fund science education and outreach at all levels, kindergarten through adult. Pittcon donates more than a million dollars a year to provide financial and administrative support for various science outreach activities including science equipment grants, research grants, scholarships and internships for students, awards to teachers and professors, and grants to public science centers, libraries and museums.
Visit pittcon.org for more information.