A new study has laid bare the structure of a protein receptor called the CysLT1 receptor that is implicated in a range of allergic and cardiovascular conditions as well as some types of cancer. Published in Science Advances, the paper shows how the researchers used structural biology techniques to accomplish this feat.
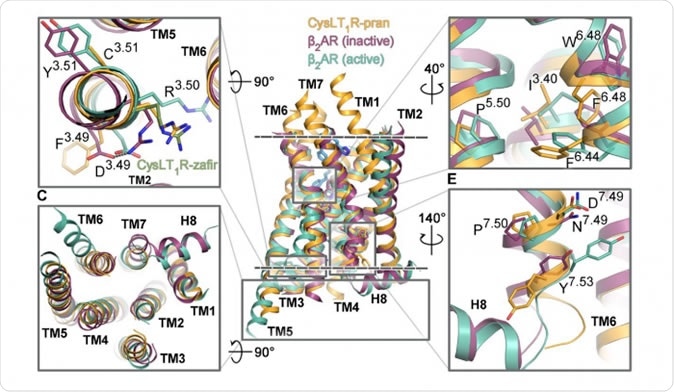
Figure 1. The segments of the CysLT1 receptor responsible for its activation are shown in orange, next to other G protein-coupled receptors. Credit: Luginina et al./Science Advances
The cysteinyl leukotriene receptor type 1 (CysLT1) receptor is part of the G protein-coupled receptor, or GPCRs, which are found as integral parts of the cell membrane. Upon activation by inflammatory molecules called leukotrienes, they transmit information from outside the cell to the inside, whether it comes in the form of light photons, molecules of fat or small proteins or DNA sequences. The result is typically the initiation of a cascade of cellular events – which can lead to cell division, migration or cell death. This signaling pathway is fundamental to cellular metabolism.
For this reason, GPCRs have been targeted by 40% of all medications in use today. However, the structure of these vital cell components was unknown, due to the difficulty and complexity of this study – until structural biologists at the MIPT Center for Molecular Mechanisms of Aging and Age-Related Diseases took it up, along with their colleagues in other nations like France, Germany, the USA and Canada. When the mechanism of operation of these receptors is known, new drugs that can selectively act upon these drugs could be developed, with less off-target actions and therefore lower toxicity.
What is structural biology?
The field of structural biology is one which crosses boundary lines to bring together physics and biology. It elucidates the structure of macromolecules in living organisms. It involves the use of genetic engineering to produce synthetic proteins, their purification and finally their crystallization. The pure crystal of protein is then dealt with on the basis of fundamental physics principles. First it is X-rayed with powerful rays to obtain the diffraction pattern, where rays bend around various atoms and groups in the protein molecule to give characteristic patterns. These are then processed via powerful and accurate mathematical models, resulting in a minute blueprint of the 3D structure of the protein, at atomic level. The model is typically accurate to angstrom level.
The X-rays used in structural biology come from two sources, synchrotons and free electron lasers. While synchrotons have been around since the 1970s, free electron lasers are quite recent, introduced less than a decade ago. Both operate on the same principle, of imparting energy to electrons to speed them up to almost the velocity of light. The next step is to redirect the course of electron motion. In a synchrotron, the path is almost circular, while with the free electron laser, they shoot through a passage formed by a double line of magnets alternately facing opposing directions – an undulator. This causes the emission of X-rays. However, the X-ray energy emitted by the free electron laser is far more powerful, allowing the diffraction to analyze tiny crystals barely a micron in diameter. Its invention has already led to the revelation of several hundred protein structures.
The CystLT1 receptor
The current study looked at the GPCR called the CystTL1 receptor that is often a participant in inflammation and allergy. It is therefore also involved in the production of asthma symptoms. Asthma affects about 10% of the population worldwide.
In order to obtain the structure of this receptor, the researchers examined its binding with two molecules called zafirlukast and pranlukast. These are used to treat patients with allergic asthma, allergic rhinitis and urticarial. Though these are known to dilate the lung airways and fight inflammation, they are not universally effective against asthma. In addition, they often cause gastrointestinal and psychiatric symptoms as side effects. The pranlukast crystals were grown to about 0.3 mm whereas zafirlukast crystals attained only a few microns diameter.
Investigations on crystals of pranlukast and zafirlukast, which were about 0.3 mm and a few microns in diameter respectively, occurred at several international centers. The researchers found the 3D structure of the complexes, extending through the entire membrane thickness. They uncovered a new disulfide bond in the CysLT1-pranlukast structure that connects two long transmembrane helices. Specific features were observed in the form of functional motifs or microswitches which change the extent of its response to leukotrienes. They were also able to identify the ligand-binding pockets in both complexes which is uniquely different from all previously observed GPCR molecules. They found various points of interaction between each of the two drug molecules with the receptor. Researcher Aleksandra Luginina says caressingly of her work, “These are unique structures, and we've grown quite fond of them.”
Implications
The structure and mechanism of the receptors changes the understanding of how GPCRs function. In addition, knowing how these drugs bind to the receptor helps to develop more selective drugs for the treatment of asthma, thereby avoiding side effects while optimizing the efficacy. All these discoveries have helped to understand how ligands bind to fat-based receptor molecules, and also show how receptors can adapt to bind two antagonists with different chemical structures.
While showing a possible mechanism of activation of the receptor, it also suggests a way to develop better drugs designed to fit the known structure as well as tools to help discover candidate molecules. The researchers can rightfully be glad that they have taken their place among the very few laboratories to have succeeded in unraveling the 3D structure of one of these receptors.
Source:
Journal reference:
Structure-based mechanism of cysteinyl leukotriene receptor inhibition by anti-asthmatic drugs. Aleksandra Luginina, Anastasiia Gusach, Egor Marin, Alexey Mishin, Rebecca Brouillette, Petr Popov, Anna Shiriaeva, Élie Besserer-Offroy, Jean-Michel Longpré, Elizaveta Lyapina, Andrii Ishchenko, Nilkanth Patel, Vitaly Polovinkin, Nadezhda Safronova, Andrey Bogorodskiy, Evelina Edelweiss, Hao Hu, Uwe Weierstall, Wei Liu, Alexander Batyuk, Valentin Gordeliy, Gye Won Han, Philippe Sarret, Vsevolod Katritch, Valentin Borshchevskiy and Vadim Cherezov. Science Advances. 09 Oct 2019: Vol. 5, no. 10, eaax2518. DOI: 10.1126/sciadv.aax2518. https://advances.sciencemag.org/content/5/10/eaax2518