Researchers in the United States have described the effectiveness of a candidate monoclonal antibody currently being evaluated in a Phase 1 trial as a therapy for coronavirus disease 2019 (COVID-19) and its causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The drug, called CPI-006, targets an immune signaling molecule called CD73 and activates CD73POS B cells, which subsequently promotes antibody production and differentiation into B cells.
In vitro, CPI-006 induces markers of B cell activation, maturation, antigen presentation, and transformation into antibody-secreting plasmablasts.
In a Phase 1 trial testing the safety and immunogenicity of CPI-006 among COVID-19 patients with mild to moderate disease, a single dose of the agent-induced antiviral antibody responses just 7 days following treatment, with antibody titers continuing to increase past day 56.
Increases in the frequency of memory B cells and effector/memory T cells were also observed 28 days after treatment.
Stephen Willingham (Corvus Pharmaceuticals, California) and colleagues from Temple University and Icahn School of Medicine at Mount Sinai say the preliminary results suggest that CPI-006 activates B cells and prolongs the antibody response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with COVID-19.
The team says CPI-006 could be useful for treating COVID-19 or as an adjuvant to improve SARS-CoV-2 vaccine efficacy.
A pre-print version of the paper is available on the medRxiv* server, while the article undergoes peer review.
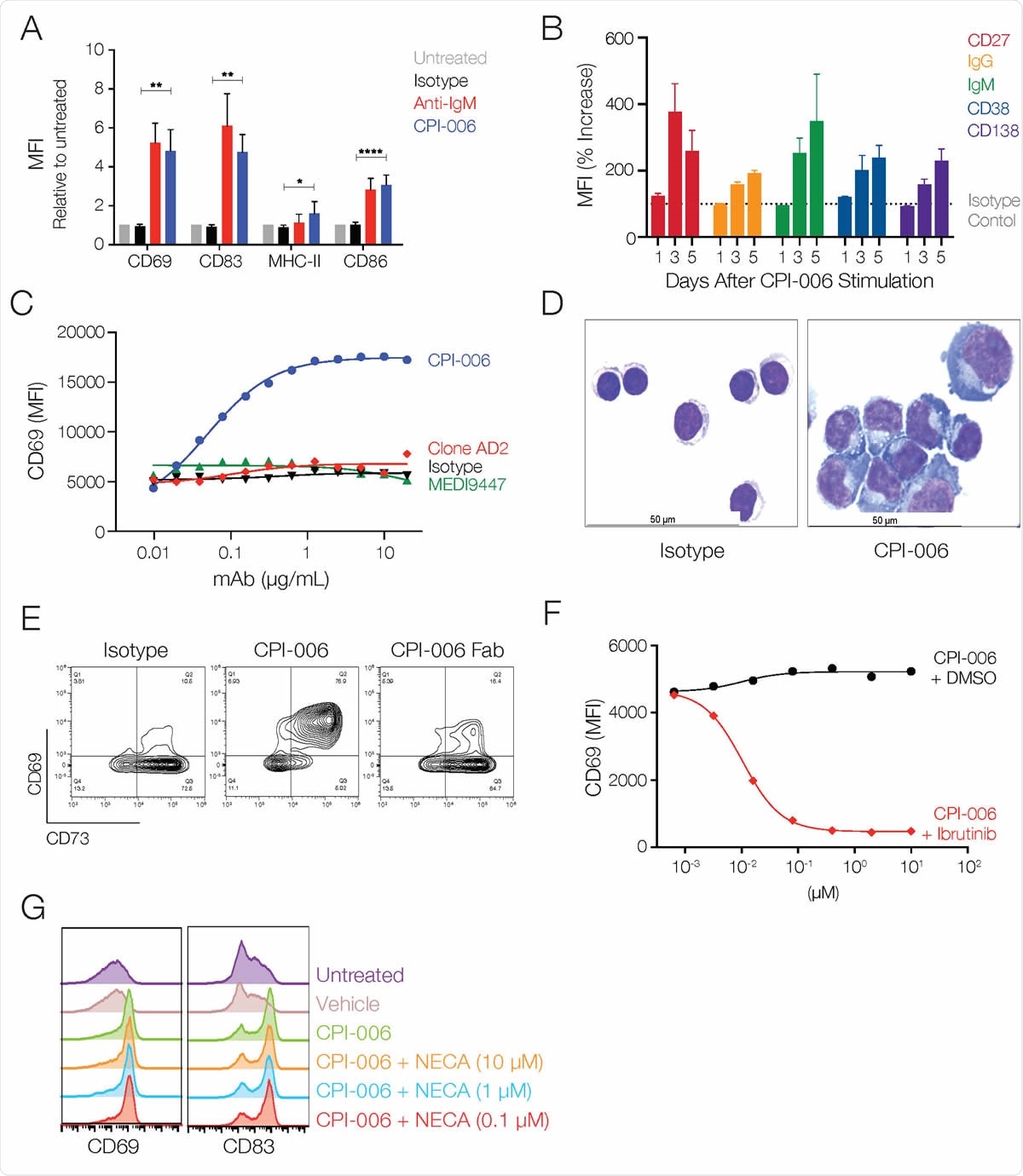
CPI-006 directly activates B lymphocytes and induces maturation into antibody secreting plasmablasts in vitro. (A) Purified B cells from 3-5 healthy donors were incubated overnight with human IgG1 isotype control or CPI-006 (10 μg/mL) or anti-IgM microbeads, a positive control for BCR stimulation. Expression of activation markers CD69, CD83, CD86, or MHC-II was measured by flow cytometry. B) Time dependent increases in the expression of CD27, IgG, IgM, CD38, and CD138 on purified B cells cultured in the presence of CPI-006 or isotype control (1 μg/mL). Mean fluorescence intensity (MFI) was determined for each marker and was normalized to the untreated or isotype control for each donor. C) B cell activation is unique to CPI-006 as other anti-CD73 antibodies do not induce CD69 expression. Expression of CD69 (MFI) was measured by flow cytometry. D) Representative images of purified B cells cultured with isotype control (left panel) or CPI-006 for 2 days. E) Purified B cells were incubated overnight with 10 μg/mL human IgG1 isotype control or CPI-006 or equimolar CPI-006 Fab. CD69 and CD73 were measured on B cells by flow cytometry. (F) Human PBMCs were incubated overnight with a fixed concentration of CPI-006 (10 μg/mL) along with ibrutinib or vehicle control over a range of concentrations. Expression of CD69 on B cells (CD19POSCD3NEG) was measured by flow cytometry. G) Human PBMCs were incubated overnight with 10 μg/mL CPI-006 with or without NECA over a range of concentrations or 10 μM APCP. Expression of CD69 on B cells (CD19+CD3-) was measured by flow cytometry and MFI is reported. Error bars represent mean ± SD. *p<0.05, **p<0.01 as determined by t-test.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Antibodies can stop SARS-CoV-2 entering host cells
To gain entry to host cells, SARS-CoV-2 uses a viral surface structure called the spike protein to bind to the human angiotensin-converting enzyme 2 (ACE2) receptor. Many recent studies have shown that antibodies that interrupt this interaction can stop this viral entry.
This suggests that antibodies specific to the spike protein or its receptor-binding domain (RBD) could be of clinical benefit to patients with COVID-19 and protect healthy individuals against infection.
CD73 is a signaling molecule found on the majority of B cells and some T cells that plays a role in lymphocyte activation and trafficking.
A role for CD73 in B cell maturation has previously been proposed, since its reduced expression on B cells among immunodeficient patients correlated with an inability to produce IgG.
However, CD73 also functions as an enzyme that converts AMP into adenosine, which can have immunosuppressive effects.
What did the researchers do?
Now, Willingham and the team have described the inhibitory effects of CPI-006 on CD73.
CPI-006 blocks the enzymatic activity of CD73, while directly activating CD73POS B cells.
This induces differentiation into plasmablasts, immunoglobulin class switching, and antibody secretion, whilst preventing the suppressive effects of adenosine on T cell proliferation and cytokine secretion.
Using flow cytometry-based screening to test the effects of treatment with CPI-006 on human B cells in vitro, the team showed that CPI-006 activated B lymphocytes and increased the cell surface expression of CD27, IgG, CD38, and CD138.
All of these markers are consistent with the induction of B cell maturation, says the team.
CPI-006 is currently being tested in a phase I study as an immunotherapy for cancer. Immunophenotypic analysis of patients who received a 3 to 24 mg/kg of CPI-006 revealed evidence of B cell activation, clonal expansion, and development of memory B cells.
“Memory B cells are essential for both acute and long-term immunity as they have undergone immunoglobulin rearrangement and somatic hypermutation in order to produce high affinity, antigen-specific antibody upon exposure to antigen,” writes the team.
Testing the effects on immune responses against SARS-CoV-2
The researchers say these observations suggested that CPI-006 may also be effective at enhancing the magnitude, diversity, and duration of humoral and cellular responses to SARS-CoV-2.
Now, the team has initiated a phase 1 safety and efficacy trial involving ten patients (aged a median of 64 years) hospitalized with mild-to-moderate COVID-19 who are receiving 0.3 mg/kg, or 1.0 mg/kg dose of CPI-006.
All evaluable patients had low levels of antibodies against the SARS-CoV-2 spike protein and its RBD, irrespective of symptom duration, prior to enrollment.
However, levels of IgG and IgM antibodies against the spike protein or its RBD rapidly increased just 7 days following a single low-dose infusion of CPI-006.
“Although CPI-006 was delivered intravenously in this study, the effects seen with low doses indicate that alternative routes of delivery such as subcutaneous or intramuscular administration are feasible,” say the authors.
Titers of neutralizing antibodies also increased, as measured by the blocking of RBD binding to ACE2.
These increased antiviral antibody responses continued to rise past day 56 following treatment, and increases in the levels of memory B cells and effector/memory T cells were also observed 28 days following treatment.
Robust and durable humoral response could improve COVID-19 outcomes
The researchers say the findings point to a robust and durable humoral response that would theoretically improve clinical outcomes in COVID-19 patients.
These effects could potentially also reduce viral transmission and expand the pool of qualified convalescent plasma donors, they add.
“These preliminary results suggest that CPI-006 activates B cells and may enhance and prolong anti-SARS-CoV-2 antibody responses in patients with COVID-19,” said Willingham and colleagues.
“This approach may be useful for treating COVID-19 or as an adjuvant to enhance the efficacy of vaccines,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Willingham S, et al. Characterization and Phase 1 Trial of a B Cell Activating Anti-CD73 Antibody for the Immunotherapy of COVID-19. medRxiv, 2020. doi: https://doi.org/10.1101/2020.09.10.20191486