As COVID-19 seems to be making a comeback in many areas of the world, including Japan, a new study published on the preprint server medRxiv* in October 2020 shows the relevance of microsamples of blood in serosurveillance of this disease.
At the beginning of April, the first wave of COVID-19 approached its peak, with the peak coming in mid-April. The total number of cases at this time was 132. Following five weeks of lockdown, the outbreak was effectively contained to zero new cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, a second wave broke two months later. The authors of the current study report that by the end of the first week in October, there had been over 2,800 cases. The study dates from the beginning of the second wave.
Importance of Serological Surveys
It is essential to conduct surveillance of infectious diseases to frame appropriate public health guidelines. One popular technique is serosurveillance to detect antibodies against SARS-CoV-2. Serosurveillance provides estimates of antibody levels against infectious diseases and is considered the gold standard for measuring population immunity due to past infection or vaccination.
The Issue with Venepuncture
The weak point of much of the world’s healthcare systems has become evident in the current pandemic: a lack of sufficient trained personnel. To diagnose SARS-CoV-2 infection, it is necessary to take a nasal swab, exposing both the healthcare worker and the patient to the added risk of transmitted infection.
The same goes for antibody testing, since venepuncture, to obtain plasma for the test sample, is a skilled procedure requiring trained staff. For both these reasons, it is vital to develop alternative ways to reduce the demand for clinical staff for this procedure and limit their exposure to potentially infected individuals who do not need their clinical expertise to alleviate suffering.
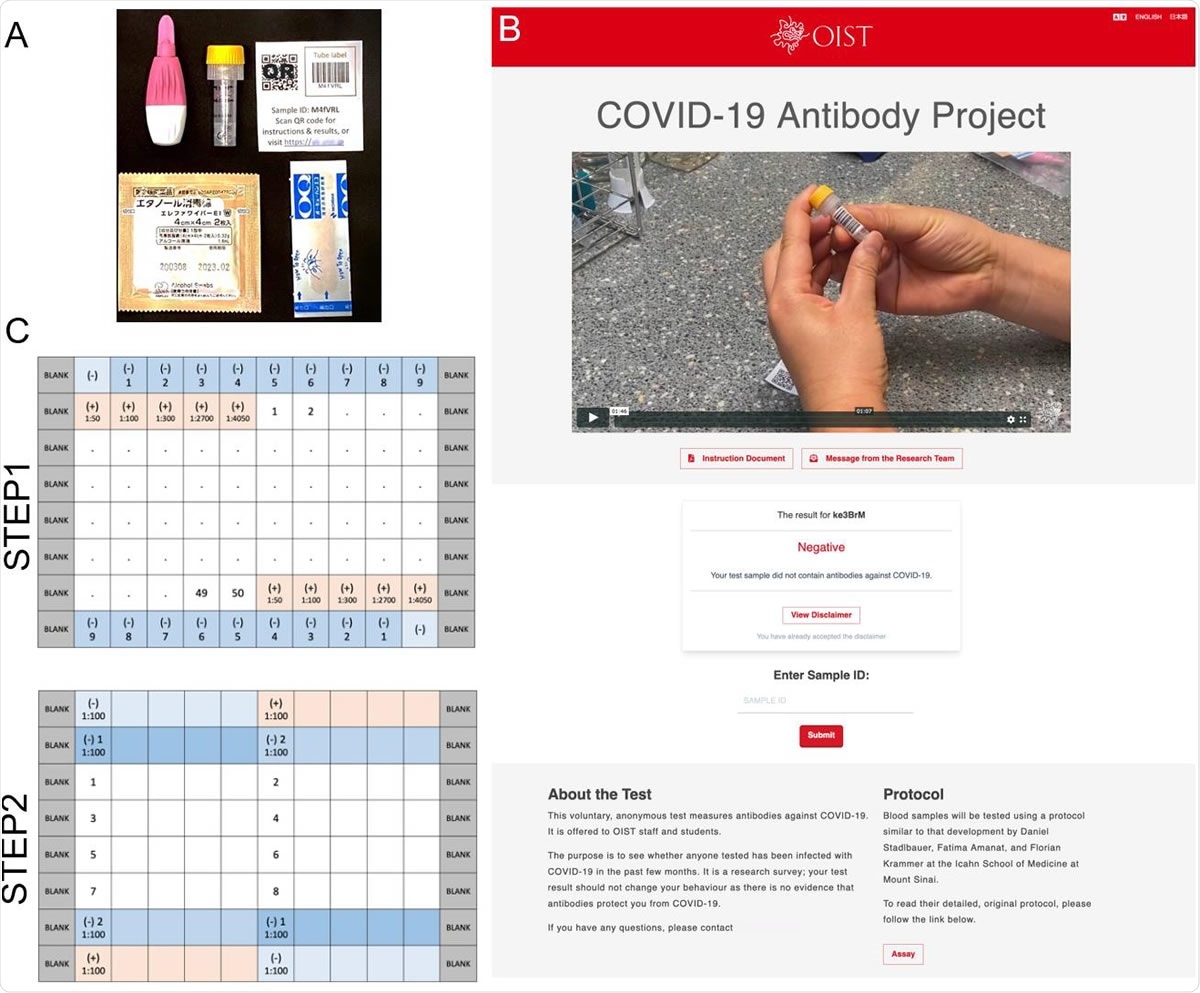
Antibody Survey Sample Collection Kit and Plate Design. A. Each blood sampling kit included BD MicrotainerⓇ 500 contact-activated lancet (Becton Dickison, USA), 0.8 ml volume blood collection tube containing a coagulant and a separator (Greiner bio-one), packaged alcohol wipes, adhesive bandage, and sticker with an identification barcode and QR code. B. Front page of OIST Antibody Test website. C. Plate designs used in the ELISA.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Exploring the Validity of Micro Blood Sampling
The current study at the Okinawa Institute of Science and Technology Graduate University (OIST), Japan, aimed to examine the feasibility of using micro blood sampling kits to allow test candidates to draw a few drops of their own blood for testing.
The researchers compared the results of a sensitive and specific ELISA against these microsamples, self-collected by the staff and students at OIST, vs. human COVID-19-positive serum samples from the local hospital and negative serum samples collected pre-pandemic. After validating the assay, they set up a partially automated workflow for rapid, anonymous handling of tracked samples.
The kit contains a disposable safety lancet that is designed to allow any individual to obtain a few and only a few drops of capillary blood by fingertip prick. The MiniCollect capillary blood collection tube used in this study is prefilled with a gel that promotes clotting so that the serum is separated from the blood clot. This serum can then be tested by ELISA using receptor-binding domain (RBD) and full-length spike antigens in two steps.
Microsampling Offers Good Alternative to Venepuncture
The use of microblood sampling, which needs only a finger prick and can be done at home, avoids the use of trained healthcare workers and the risk of infection while also making it possible to collect samples on a large scale with great rapidity. Even though some participants were not able to collect the samples by themselves, requiring the help of a trained healthcare worker, the routine use of this modality of the collection could hugely reduce the load on and the risk to the healthcare staff.
When these self-collected capillary blood microsamples are tested by a high-quality ELISA at one center, using as little as one drop, or 30 μL per sample, the results will be very accurate. At the same time, the cost of screening a large population is kept low.
Microsampling is Compatible with Routine Mail Shipping
The assay is not time-dependent since serum can be separated from whole blood after up to a week after collection, and blood does not deteriorate at room temperature within this period. This allows for samples to be shipped to the testing laboratory by ordinary mail.
In order to allow the rapid loading of numerous serum samples on to the ELISA plate, the researchers used a robotic liquid handling system.
Antibody Titers Comparable for Microsamples and Venous Blood Samples
The researchers received 675 samples in all. Of these, ~6% were unusable, being insufficient or of low quality. Of these, 63 samples were reactive for RBD in step 1 of the assay, but none for the trimeric spike protein in step 2. This indicates a ~10% false-positive rate. When the assay was tested with serum from individuals known to be PCR-positive, all the samples were reactive in both steps of the assay.
The researchers compared antibody titers measured from two people who had tested positive for SARS-CoV-2 in two samples each, one of venous blood and the other by finger prick. Both patients were 92 days or more from exposure, but had a high antibody titer similar to that of controls, despite the latter having been collected within a month of exposure.
These findings fill a significant gap in the reliability of capillary blood use in serological assays and may promote further research in using this method in other studies.
Cross-Reactivity with MERS Convalescent Serum
The antibodies found in samples of SARS-CoV plasma are moderately cross-reactive with SARS-CoV-2 spike protein, though they cannot neutralize the latter virus. The ELISA used in the current study also showed cross-reactive antibodies in plasma from convalescent MERS patients when exposed to the SARS-CoV-2 spike protein. Interestingly, the antibody titer was similar in both MERS and COVID-19 patients.
The sequence identity between the RBD of SARS-CoV-2 and MERS-CoV was less than 20%, whereas for the spike protein, it was over 30%. This increased conservation of spike domains outside the RBD may account for the presence of these cross-reactive antibodies.
Rapid Anonymous Reporting
The researchers used a web-based platform to carry out anonymous sampling and reporting, from passing on instructions to providing the results to the participants. The samples were barcoded to allow anonymous tracking, and thus the use of personal data was avoided.
Results and their Significance
The survey showed zero seroconversion in this small population. However, it accurately detected all those who were known to be PCR-positive by prior testing, and also the controls who were not infected.
Other surveys have shown a low seroprevalence in Okinawa, the location of the current study, and in Japan, overall, varying from 0.03% to 0.43% in different regions. In such settings, the testing method used must have high specificity. That is, it must accurately report a very high percentage of uninfected individuals as tested negative.
In this context, and given the ~100% specificity and ~93% sensitivity of the ELISA assay used here, the negative predictive value of the current assay is almost 100%. In other words, a negative result is almost certainly indicative of the absence of SARS-CoV-2 infection in the tested individual.
Implications
The study shows that capillary blood provides reliable results for antibody testing and can replace venous blood drawn by venepuncture. This paves the way for inexpensive, simple, and rapidly scalable serological testing. When used with a high-quality ELISA such as that developed by these researchers in a centralized testing lab, it is an optimal solution to the problem of large-scale serological surveillance of larger populations.
Again, if the titer of anti-spike antibodies in a given specimen of SARS-CoV-2 convalescent serum is 1:1,350 or higher, the odds of successful viral neutralization with convalescent plasma, used according to FDA recommendations, are 80% or more, and at this level, both MERS and SARS-CoV-2 antibodies show potent cross-reactivity. This agrees with the detection of neutralizing antibodies targeting the NTD of the SARS-CoV-2, even though the majority are directed against the RBD. This also supports the use of MERS convalescent serum to treat COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources