EKF Diagnostics, the global in-vitro diagnostics company, announces that it has launched a PrimeStore® MTM sample collection kit. This new kit enables the convenient and safe collection, transportation, and handling of pathogenic samples, including COVID-19 and influenza.
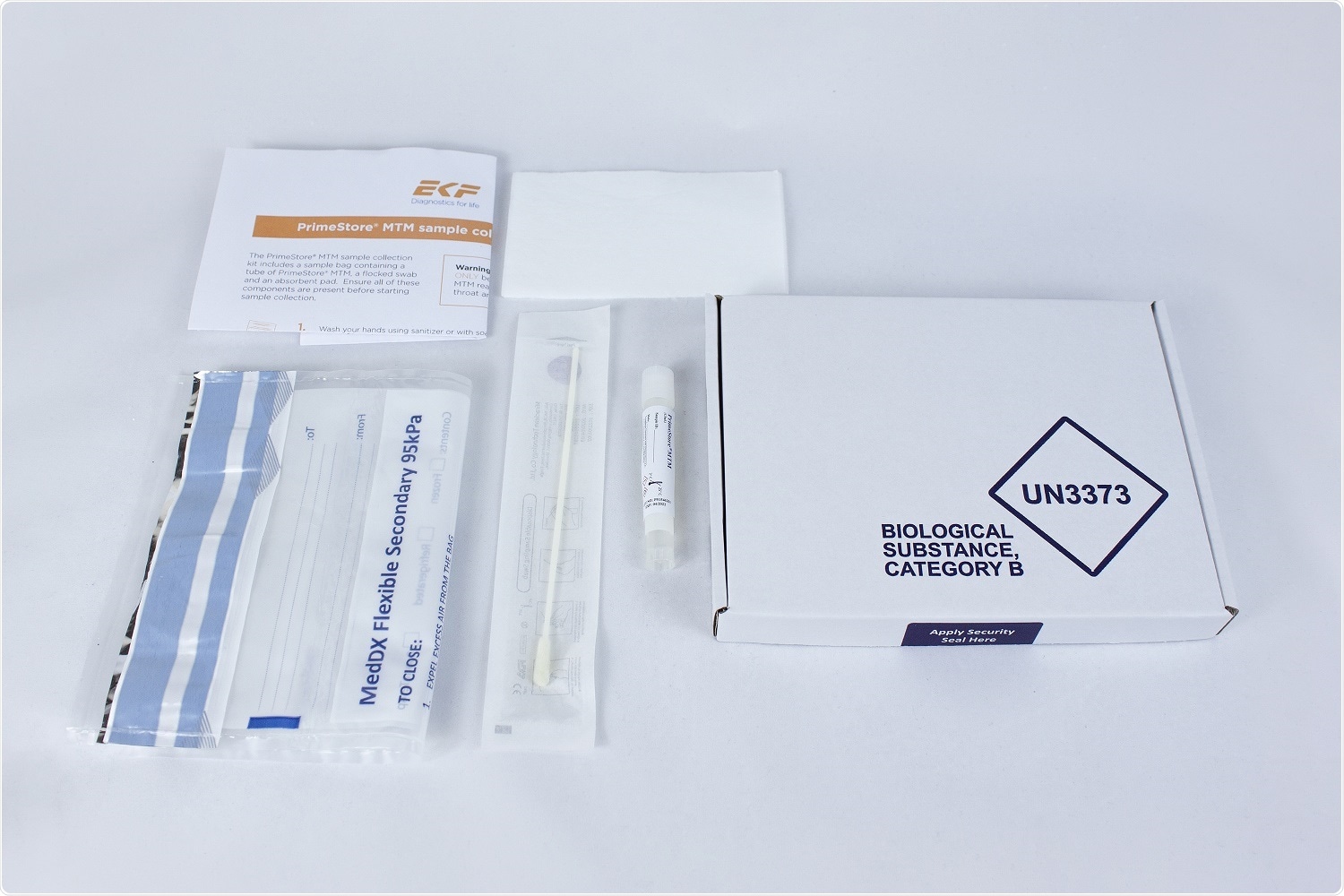
Image Credit: EKF Diagnostics
Two kit configurations are now available: a postal sample kit ideal for home use and a multi-pack of sample kits suitable for on-site mass sampling. To meet the growing demand for this sample collection device, EKF has increased its manufacturing capacity in the UK and mainland Europe.
Pathogenic samples stored in the FDA-cleared, CE-IVD marked PrimeStore MTM novel viral transport media, including SARS-CoV-2, are inactivated within minutes of collection. This allows such samples to be transported safely through the postal system or via courier and complies with UN3373 packaging regulations. Furthermore, as samples are stable in PrimeStore MTM for seven days at ambient temperatures, this removes the need for all cold chain logistics during sample collection, transportation, and storage. Thereby, preventing mishandled samples and making the postal collection a realistic option.
Multiple nucleic acid species can be tested from one sample as RNA and DNA are perfectly preserved and stabilized by PrimeStore MTM for downstream molecular applications. Furthermore, because PrimeStore MTM totally inactivates SARS-CoV-2, as demonstrated by a recent Public Health England evaluation study, the risk of infection to lab staff from samples is eliminated. This means that samples are not only safe during transportation, but also ready for immediate safe testing at a laboratory with no need for containment.
The new sample collection kit pairs PrimeStore molecular transport media in a cryovial with a sterile flocked swab (for nasal and oral sampling) in a CE marked kit, which also includes a UN3373 95kPa compliant specimen collection bag, absorbent pad, and instructions for use. In addition, the postal sample collection kit includes a UN3373 compliant postal box with a security seal for sample return and a postal pouch for collection kit delivery.
Due to the current COVID-19 pandemic, EKF has received a number of new orders for PrimeStore MTM sample collection devices; including initial orders from two new customers, the Health Service Executive (HSE), Ireland, and a national health body in England. These add to the growing demand for PrimeStore MTM from healthcare, education, and industry to supply safe sample collection kits for COVID-19 testing programs.
To ensure that it can fulfill the increasing orders for PrimeStore MTM and the new sample collection kit, EKF is ramping up manufacture in the UK and also mainland Europe. The Company has now started production at its facilities in Barleben, Germany, and will soon be moving its current Penarth-based manufacturing lines in Wales to a new larger facility in Llandough, Cardiff, which is over 600 sq. meters in size. This represents a 100% increase in the manufacturing area.
For more information on EKF Diagnostics, see www.ekfdiagnostics.com.