As the number of coronavirus disease 2019 (COVID-19) cases mount worldwide, the acute illness has been observed to be associated with thromboembolic phenomena.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Both the immune and the clotting systems are thrown into disarray following infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. Lymphopenia specifically affecting the T cells has been found to persist for months, while coagulation defects precipitate severe and widespread organ damage in many severely ill COVID-19 patients.
Factor V and severe COVID-19
Patients with acute severe COVID-19 have a substantial increase in factor V levels, and an associated higher risk of thromboembolism. This factor is known to be secreted by T cells and monocytes. In particular, T regulatory cells (Tregs) are found to be a prominent source of factor V in these patients, just as in healthy blood donors. The production of factor V by peripheral blood mononuclear cells did not seem to be associated with the severity of disease, however.
Factor V is produced by neutrophils
The current study identifies neutrophils as another source in patients with severe COVID-19. The expression of factor V was closely linked to that of other genes expressed strongly in neutrophils. While this factor V gene module was not overexpressed in asymptomatic or mildly symptomatic individuals, it was highly expressed in severe COVID-19 and continued to be elevated for weeks thereafter. Most other coagulation factors failed to follow this pattern.
The study also suggests that this factor plays a key role in disrupting the normal immune response in severe COVID-19. Genes encoding factor V were upregulated in circulating blood cells – albeit modestly – and this increase was linked to plasma factor V levels.
The important contribution to the regulation of the immune response to the virus is, however, probably made by leukocyte-derived factor V secreted from the cells that have undergone migration into the tissues. Interestingly, this includes leukocyte migration into secondary lymphoid organs, which are sites normally protected against the effects of circulating factor V.
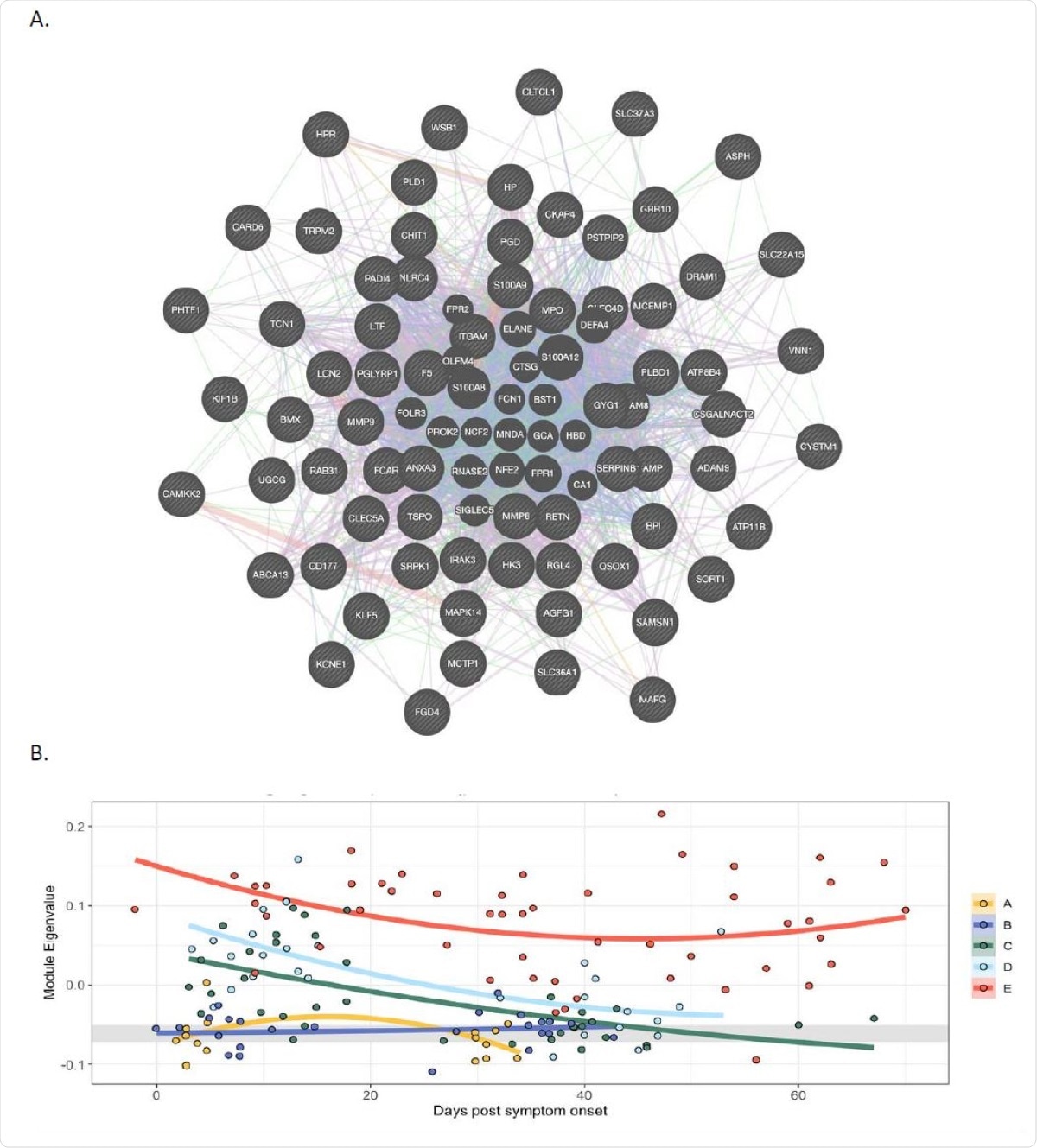
In peripheral blood cells FV is co-expressed with a module of genes expressed in neutrophils, and expression of the module correlates with severity of Covid-19 A. Weighted gene co-expression network analysis identified a module containing group of genes co366 expressed with FV. In this module FV is a hub gene and its expression correlates strongly with genes expressed in neutrophils. The lines are colour coded to show relationships. Purple: Gene known to be co-expressed in existing gene databases. Orange: Predicted functional relationships between genes. Pink: Proteins known to be linked. Turquoise: Genes present in a shared annotated pathway. Blue: Genes expressed in the same tissue. B. Mixed-effects model with quadratic time trend showing the longitudinal expression of the FV module over time, grouped by severity. Grey band indicates the interquartile range of HCs. A significant effect of time versus severity group interaction term (p = 0.0047) indicates that disease severity has a significant effect on longitudinal expression. A, HCW screening asymptomatic; B, HCW screening symptomatic; C, hospitalised mild disease; D, hospitalised requiring oxygen; E, hospitalised, intensive care.
Factor V suppresses T cell proliferation
The researchers found that factor V suppresses the proliferation of T cells in vitro. However, factor V activated by thrombin does not show this suppressive effect. The use of a recombinant factor V with a mutation that causes prevents its activation by thrombin leads to greater suppression of T cell proliferation.
Severe lymphopenia in COVID-19
Patients with COVID-19 could have a higher expression of factor V in the blood, associated with the suppression of T cell counts. This appears to persist in the blood for months following SARS-CoV-2 infection.
Bronchoalveolar lavage fluid (BALF) from patients with severe COVID-19 contained many neutrophils, monocytes, macrophages, and eosinophils. Surprisingly few lymphocytes were observed, especially the effector T cells, both CD4 and CD8. Tregs increased in proportion.
Secondly, severe lymphopenia is observed in lung areas affected by SARS-CoV-2 pneumonia, which show macrophage or neutrophil infilrates or display neutrophil extracellular traps (NETs). It appears, therefore, that leukocyte-derived factor V may cause T cell suppression at infection sites.
Supporting this hypothesis, the researchers also observed that factor V levels in plasma were linked to the levels of coagulation. At the same time, T cell counts were associated with the level of expression of factor V genes in circulating white cells. Most of the plasma factor V comes from the liver, which is a site of adaptive immune tolerance.
What are the implications?
The study demonstrates that the elevation of factor V levels in plasma in patients with severe COVID-19 is due to overexpression by circulating neutrophils, Tregs and monocytes. The high factor V levels cause T cell suppression. Activation of factor V by thrombin causes this immunosuppressive activity to vanish.
While all hospitalized COVID-19 patients typically receive VTE prophylaxis, the researchers suggest that this may have unexpected consequences. Among the anticoagulants used currently to prevent venous thromboembolism (VTE) in COVID-19, those, like heparin, which have anti-thrombin activity, may reduce the activation of factor V. By so doing, it can simultaneously enhance the factor-V dependent suppression of T cells.
In fact, heparin has been experimentally shown to have an immunosuppressive effect, particularly on T cells. In severe COVID-19, the high factor V levels, produced by immune cells, may be responsible for severe lymphopenia and suppression of the adaptive immune response, and heparin may exacerbate this situation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Wang, J. et al. (2021). Factor V is an immune inhibitor that is expressed at increased levels in leukocytes of patients with severe Covid-19. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.14.21249801, https://www.medrxiv.org/content/10.1101/2021.01.14.21249801v1
- Peer reviewed and published scientific report.
Wang, Jun, Prasanti Kotagiri, Paul A. Lyons, Rafia S. Al-Lamki, Federica Mescia, Laura Bergamaschi, Lorinda Turner, et al. 2022. “Coagulation Factor v Is a T-Cell Inhibitor Expressed by Leukocytes in COVID-19.” IScience 25 (3): 103971. https://doi.org/10.1016/j.isci.2022.103971. https://www.cell.com/iscience/fulltext/S2589-0042(22)00241-3.