Scientists across the globe are fighting to contain the ongoing coronavirus 2019 (COVID-19) pandemic. They are conducting extensive research to understand the characteristic features and mode of action of the causal organism, namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Further, the duration for which the anti-SARS-CoV-2 antibodies persist in the body of recovered patients is an important aspect on which several critical policies such as vaccination programs depend.
Several hypotheses regarding fading of humoral immunity in COVID-19 recovered patients exist. The waning of antibodies has raised multiple concerns within the scientific community as antibodies provide long-term protection against reinfection and the durability of vaccine-induced protection.
Previous studies have indicated that the COVID-19 infection induces the production of protective neutralizing antibodies. However, some studies have claimed a rapid decline of SARS-CoV-2 antibodies, i.e., three months after infection, while few studies have revealed persistence of antibodies for five months post-infection.
Interestingly, a recent study has reported that 95 percent of the studied candidates had retained substantial immune memory six months post COVID-19 infection. This study had also claimed that the presence of SARS-CoV-2 anti-nucleocapsid (N) and anti-spike (S) IgG antibodies could reduce risks of reinfection up to seven months post-infection. However, the emergence of highly virulent SARS-CoV-2 variants has raised further questions on whether the antibodies generated during the initial infection could protect an individual from reinfection by SARS-CoV-2 variants.
Researchers believe that for the analysis of the overall evolution of the COVID-19 pandemic, amidst the emergence of SARS-CoV-2 variants, and post-pandemic dynamics, evaluation of long-term efficacy of the immune response is highly important.
A new study has been published on the medRxiv* preprint server which focuses on validating the serological assays using a large cohort of healthcare workers (HCWs) from Strasbourg University Hospital, France, who recovered from mild COVID-19 infection. They studied the SARS-CoV-2 humoral response for thirteen months post-infection. Further, the frequency of reinfection within the above-stated study period was determined. An S-Fuse live-virus neutralization assay was also conducted to evaluate the sensitivity of SARS-CoV-2 variants against anti-S antibodies before and after vaccination.
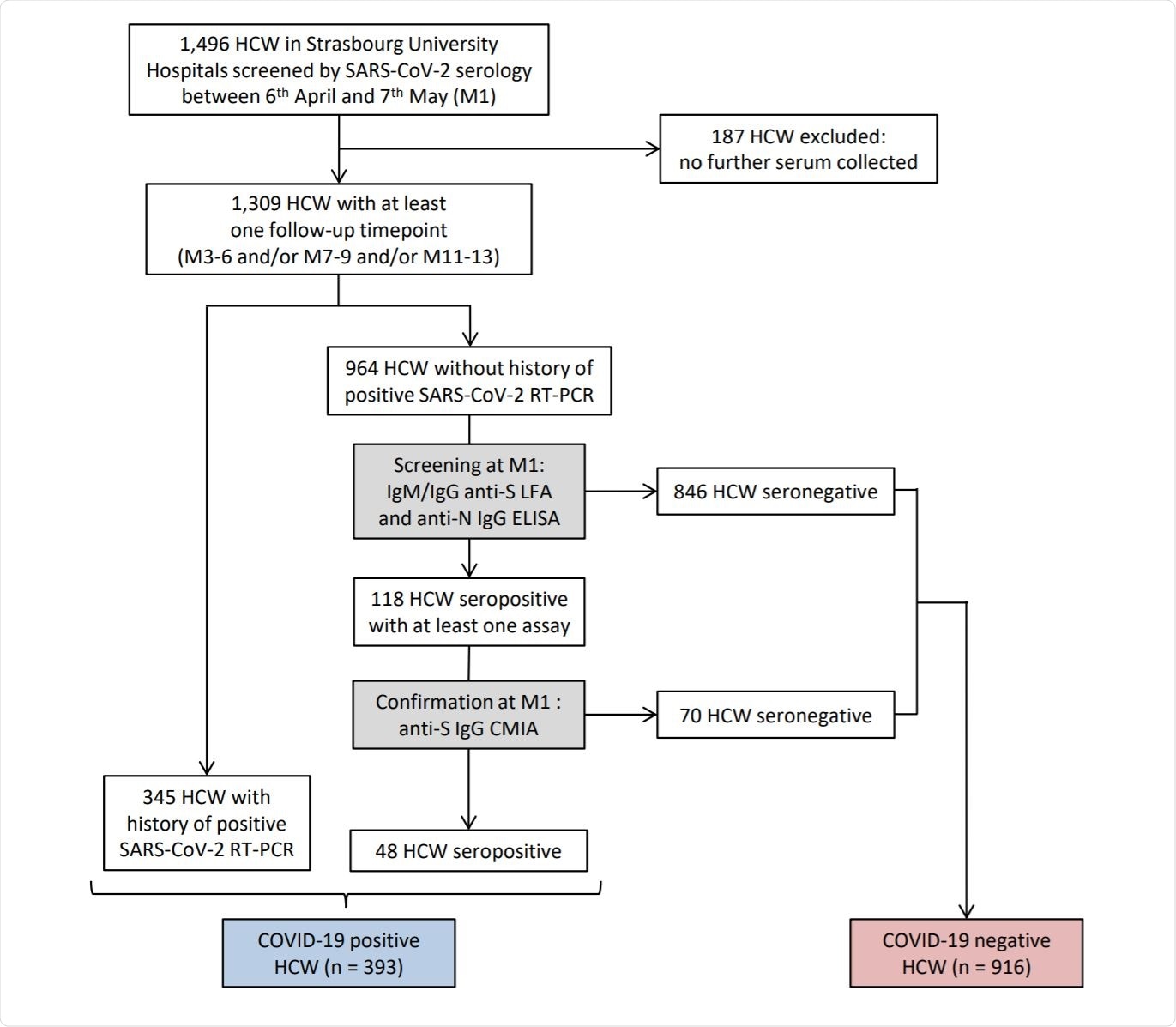
Flow chart of survey recruitment and serum sampling among the healthcare workers (HCWs) at Strasbourg University Hospital. Image Credit: https://www.medrxiv.org/content/10.1101/2021.05.07.21256823v2.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In this study, researchers recruited 1,496 HCWs based on serology screening or by a previous RT-PCR, between 6th April and 7th May 2020. All the selected candidates were categorized into M1, M3-6, M7-9, and M11-13, where M1 is defined as one-month post-inclusion. Additionally, candidates with no history of COVID-19 infection or negative RT-PCR were recruited for assessing the incidence of infection. Every participant of the study answered a questionnaire containing questions about sociodemographic characteristics, virological findings, symptoms, and vaccination after each visit.
In comparison to an earlier longitudinal study that had reported the presence of antibody titers for eight months following the infection, the present study has shown its presence for one year post-infection. The current research revealed a tri-phasic decay of anti-S antibodies. Further, individuals who received a single dose of the SARS-COV-2 vaccine, regardless of the type of vaccine they received (mRNA or adenovirus-vector vaccines), possessed a high antibody titer that could neutralize all three variants tested in this study. The increase in the levels of antibodies was observed 7 days after vaccination.
Therefore, scientists marked a strong memory B cell response in COVID-19 convalescents, even in individuals with low antibody levels. This report is in line with an earlier study that dealt with the characterization of memory B cells and reported an initial marginal drop in the level of antibodies in COVID-19 recovered individuals, which did not reflect the waning of humoral immunity. During this phase, antibody maturation occurs and the presence of anti-S memory B cells has been reported.
The outcomes of the current study are listed below:
- Differential evolution of anti-SARS-CoV-2 antibody titers in men and women. Faster decay of anti-S IgG was reported in men.
- One year after the onset of the COVID-19 symptoms, anti-S IgG stabilize at a median titer of 2.39 log AU/mL (IQR: 2.10 – 2.75).
- Reduction in the probability of SARS-CoV-2 reinfection by 96.7% twelve months after the initial infection.
- CMIA anti-S IgG titers are associated with neutralization titers.
- Efficient neutralization of SARS-CoV-2 variants, namely, D614G, B.1.1.7 by anti-S IgG titers around 2.3 log AU/mL efficiently, but not B.1.351 variant
- The COVID-19 vaccine considerably enhanced the concentration of anti-S antibodies that neutralize all three variants irrespective of pre-vaccination IgG levels, number of doses, or type of vaccine.
- Even though Rh- status of the blood group was found to be correlated with faster decay of anti-S IgG titers with time, no relation was observed with ABO blood groups.
One of the limitations of the current study is that the neutralization experiments were conducted using a small sample. Further, the assessment of reinfection of COVID-19 convalescents was based on the reports of the participants during their routine visits and no RT-PCR surveillance was performed. A third limitation is the unequal sex distribution, i.e., the number of female candidates was more than male candidates. Lastly, owing to the lack of peripheral blood mononuclear cells, researchers were not able to analyze the kinetics of memory B cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gallais, F. et al. (2021). Anti-SARS-CoV-2 Antibodies Persist for up to 13 Months and Reduce Risk of Reinfection. medRxiv 2021.05.07.21256823;
doi: https://doi.org/10.1101/2021.05.07.21256823
- Peer reviewed and published scientific report.
Gallais, Floriane, Pierre Gantner, Timothée Bruel, Aurélie Velay, Delphine Planas, Marie-Josée Wendling, Sophie Bayer, et al. 2021. “Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection.” EBioMedicine 71 (September). https://doi.org/10.1016/j.ebiom.2021.103561. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00354-6/fulltext.