Dry Powder Inhalers (DPIs) are employed to supply a safe dose of Active Pharmaceutical Ingredient (API) to the deep lung.
Image Credit: Freeman Technology
An excipient transports the delicate particles of the API from the capsule until it is trapped in the throat and then swallowed as the API travels to the lungs.
The performance of the DPI is reliant on multiple properties of the powder, such as friction, inter-particular mechanical locking, permeability and its response to air.
The characteristics of generic products can be correlated to their branded counterparts by analyzing the rheological features of DPI formulations.
Image Credit: Freeman Technology
It is also essential that the capsule filling technique and the inhaler mechanism and geometry are alike because any differences in the operation or production methods could cause changes in efficacy and performance.
Process Relevant Powder Characterization
The amount of powder that is supplied to the deep lung, the delivered dose, must be managed for DPIs. For the transport of the API into the deep lung to be successful, it is essential that the powder distributes in the inhaled air stream.
The aeration characteristics of the formulation are crucial, for example the powder’s response to the introduction of air, the cohesive forces between powder particles, and the ability of the powder to fluidize.
It is normally a requirement to perform a range of thorough laboratory testing in order to quantify the Fine Particle Dose (FPD), for example the mass of particles smaller than 5 µm.
In previous investigations, FPD has been demonstrated to have a strong correlation with features such as permeability, Aerated Energy (AE), and compressibility1,2. Figure 1 presents an example of this correlation for AE.
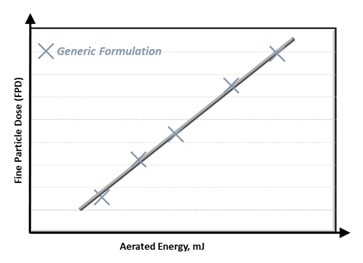
Figure 1: Fine Particle Dose (FPD) correlates with Aerated Energy. Image Credit: Freeman Technology
The AE can be compared to that of the Branded Formulation when a new Generic Formulation is created, as displayed in Figure 2.
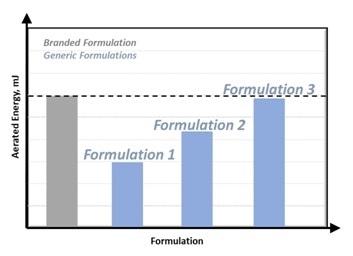
Figure 2: Matching Generic Formulation properties to Branded Formulation. Image Credit: Freeman Technology
By changing the formulation as required, such as increasing the fines content, the AE of the Generic Formulation can be correlated to the Branded Formulation to verify that the performance will be the same in the application.
When generic DPI formulations are matched to branded products, manufacturers can be more confident in creating formulations that will have features that support a comparable performance, for example the successful transport of API to the lungs and uniform dosage.
References and Further Reading
- Kinnunen et al., AAPS PharmSciTec, 2014.
- Pitchayajittipong et al., Int. J. Pharm., 2010.
About Freeman Technology
Freeman Technology specialises in systems for measuring the flow properties of powders and has over 15 years’ experience in powder flow and powder characterisation. The company invests significantly in R&D and applications development, and provides detailed know-how to support its range of products. Expert teams guide and support users around the world in addressing their individual powder challenges, focusing on delivering the most relevant information for the process. The result is world-leading solutions for understanding powder behaviour - in development, formulation, scale-up, processing, quality control, or anywhere that powders have a role.
Freeman Technology’s solutions include the FT4 Powder Rheometer®, a uniquely universal powder tester, and the Uniaxial Powder Tester, a complementary tool for quick and robust powder assessment. Systems are installed around the world in the chemical, pharmaceutical, toners, foods, powder coatings, metals, ceramics, cosmetics, and many other industries. They deliver data that maximise process and product understanding, accelerating R&D and formulation towards successful commercialisation, and supporting the long term optimisation of powder processes.
Freeman Technology is also the global distributor for Lenterra sensor instrumentation which provides in-line, real-time flow measurement solutions to enhance process understanding, and improve manufacturing efficiency and quality.
Founded in 1989 as a developer of automated testing systems for materials characterisation, the company has focused exclusively on powders since the late 1990s and in 2018 became part of Micromeritics Instrument Corporation The company’s R&D, manufacturing and commercial headquarters are in Gloucestershire, UK, with operations and distribution partners in key global territories.
In 2007 the company received the Queen’s Award for Enterprise in Innovation and in 2012 the Queen’s Award for Enterprise in International Trade.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.