Running samples on any chromatography instrument involves sample preparation as a fundamental process. For even the highest quality instruments, sample clean-up is crucial in obtaining the best sensitivity and reproducibility. One traditional method frequently used for sample clean-up is liquid-liquid extraction (LLE), which enables the transfer of analytes to an organic solution from an aqueous one. LLE can, however, present issues around time, irreproducibility and the formation of emulsions.
This application note details the benefits that can arise from using a supported liquid extraction (SLE) instead of an LLE method for extracting a range of analytes (acidic, basic and neutral) from pig plasma. SLE provides an efficient alternative method to LLE, which follows the same principles of LLE methods. It improves reproducibility and recovery, as well as speeding up sample preparation to allow greater throughput of samples tested.
Introduction
Liquid-liquid extraction was one of the first and is now one of the most established methods of sample preparation. LLE was first developed in 1909 by the petroleum industry with the aim of removing aromatic hydrocarbons from kerosene.1 The principles underlying LLE are well known and the number of publications simplifies finding a method.
LLE employs two different solvent phases, which are immiscible with each other - typically a water immiscible solvent (e.g. hexane, dichloromethane, ethyl acetate) along with an aqueous solution. Shaking is then used to drive the compound of interest from one phase to the other, usually from an aqueous to an organic solution. The partition coefficient, Kp, calculated using Equation 1, is a measure of the efficiency at which this occurs.
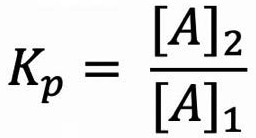
Equation 1. Equation to calculate the partition coefficient, Kp, where [A]1 is the concentration of analyte A in phase 1 and [A]2 is the concentration of analyte A in phase 2. Image Credit: Porvair Sciences Limited
The partition coefficient is known as ‘Log P of a molecule’ in chromatography, which applies the logarithm of Equation 1 when phase 1 is deionized water and phase 2 is n-octanol. This can give information on how hydrophobic/lipophilic a molecule is - a useful measure for chromatography method development.
A low Log P means the compound is more hydrophilic, making it more difficult to extract from aqueous solutions.
LLE has become a popular extraction method in chromatography laboratories due to the basic principles of solvent separation and easy access to common solvents, glassware, and equipment. There are, however, significant disadvantages to this method.
Shaking is a crucial step in obtaining efficient partitioning between the phases. The action of shaking creates an increased surface area contact between the two solutions and allows a much better transfer of analyte between phases. If this mixing is not sufficient, then the LLE method will become inefficient. On the other hand, too vigorous agitation can cause the mixture to form an emulsion- the formation of droplets of one solvent in the other, which occurs when there are surfactants present. The surfactant facilitates interaction between the two phases which can cause an intermediate phase on the surface boundary (Figure 1).
LLE is also considered low throughput as samples are prepared serially instead of in parallel. Each step of the LLE process requires repeated agitation and transfer of solvent, thus decreasing the number of samples that may be simultaneously processed. These steps are also affected by different lab users, which may cause variations in results.
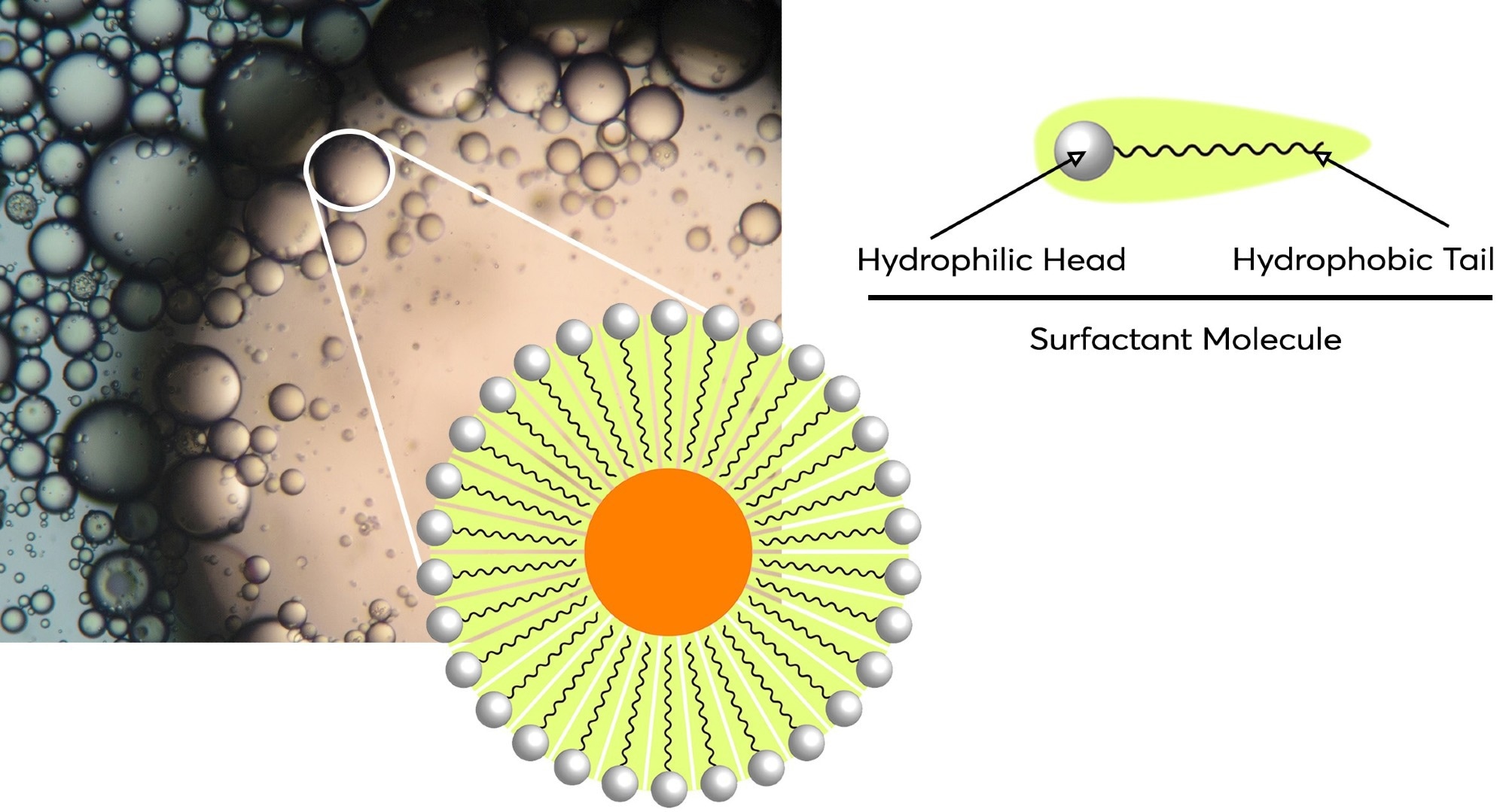
Figure 1. Diagram of a micelle which has formed part of an emulsion. The formation of them is due to the amphipathic nature of surfactants present which have both hydrophilic heads and hydrophobic tails. Image Credit: Porvair Sciences Limited
SLE, The evolved method
SLE is another sample preparation method that is based on the same principles as LLE. In SLE, diatomaceous earth is used as the support for the separation process to occur on. The material occurs naturally and is a porous, chemically inert material with a high surface area. These properties enable water to easily load via capillary action onto the diatomaceous earth and adsorb to the surface of the diatom structures of which it is made (Figure 2).
The SLE process mimics LLE theory in that two liquid phases interact with each other. Phase one is formed when the aqueous solution (water, plasma, serum etc.) is loaded and free to flow via capillary action for a period of several minutes. This enables the adsorption of the water onto the surface of the diatomaceous earth, creating a phase that creates a very large surface area.
Phase 2 is a water-immiscible organic solvent passed through the support bed under gravity. A partition with a very efficient phase boundary is formed as the solvent flows past the adsorbed water, acting like the shaking step in LLE. It allows sample clean-up as unwanted compounds remain dissolved in the adsorbed water. These include compounds such as polar contaminants or phospholipids.

Figure 2. Diatomaceous earth (DE) is composed of naturally occurring silica-based mineral made from fossilized diatoms, a class of hard-shelled algae found in seas and oceans. Its small pore size and high surface area makes it the ideal material for absorption of aqueous solutions. Image Credit: Porvair Sciences Limited
SLE is widely regarded as the more reproducible method of the two, from a sample-to-sample, experiment- to-experiment and analyst-to-analyst basis. It mitigates the need for variable steps (shaking, manual handling and throughput) associated with LLE.
The need for shaking and manual extraction of the solvent layer are also eliminated as a result of efficient interactions via solvent flow under gravity. SLE can also be easily automated by combining 96 well plates and robotic samplers, allowing a great number of samples to be processed in a single day, enabling higher throughput of samples. In this application note, the advantages of SLE versus LLE will be demonstrated regarding both performance and time.
Instrument methods
Table 1. LC system conditions for chromatographic separation of basic, acidic and neutral analytes. Source: Porvair Sciences Limited
| Basic Analyte LC Conditions |
|
| Column temp. |
45 °C |
| Injection volume |
2 μL |
| Flow rate |
600 μL/min |
| Mobile phase A |
0.1% Formic acid in water |
| Mobile phase B |
0.1% Formic acid in methanol |
| Solvent Composition |
| Time (min) |
A% |
B% |
| 0.00 |
95.0 |
5.0 |
| 4.30 |
57.5 |
42.5 |
| 7.00 |
57.5 |
42.5 |
| 7.01 |
95.0 |
5.0 |
| 10.0 |
95.0 |
5.0 |
|
| Acidic Analyte LC Conditions |
|
| Column temp. |
30 °C |
| Injection volume |
2 μL |
| Flow rate |
400 μL/min |
| Mobile phase A |
0.1% Formic acid in water |
| Mobile phase B |
0.1% Formic acid in methanol |
| Solvent Composition |
| Time (min) |
A% |
B% |
| 0.00 |
45.0 |
55.0 |
|
| Neutral Analyte LC Conditions |
|
| Column temp. |
30 °C |
| Injection volume |
2 μL |
| Flow rate |
400 μL/min |
| Mobile phase A |
0.1% Formic acid in water |
| Mobile phase B |
0.1% Formic acid in methanol |
| Solvent Composition |
| Time (min) |
A% |
B% |
| 0.00 |
70.0 |
30.0 |
| 5.10 |
25.0 |
75.0 |
| 6.00 |
25.0 |
75.0 |
| 6.01 |
70.0 |
30.0 |
| 12.00 |
70.0 |
30.0 |
|
Table 2. Mass spectrometry parameters for all methods. Source: Porvair Sciences Limited
| MS Conditions |
|
| Gas Temperature |
350 °C |
| Gas Flow |
13 L/min |
| Nebulizer |
30 psi |
| Capillary Voltage |
4000 V |
| Fragmentor Voltage |
100 V |
| Scan Type |
SIM |
| Ion Mode |
ESI |
LC system
Agilent LC-MS, made up of a 1260 LC and Single Quadrupole Mass Spectrometer. Computer running chromatographic software (OpenLabs)
Column
Raptor Biphenyl 30 x 2.1mm, 1.8μm
Table 3. Properties for the compounds analysed - a Value from Drugbank b Predicted value from Pubchem. *Compound has multiple ionisable groups present. Source: Porvair Sciences Limited
| Compound |
Compound Class |
Formula |
Molecular Mass |
LogP[12] |
pKa[9] |
Extraction Solvent |
| Caffeine |
Neutral |
C8H10N4O2 |
194.2 |
-0.07a |
14.00 |
Ethyl acetate |
| Procainamide |
Base |
C13H21N3O |
235.3 |
0.88a |
9.32 |
3:1 Hexane:Ethyl acetate |
| Acetaminophen |
Neutral |
C8H9NO2 |
151.2 |
0.91a |
-4.40 |
Ethyl acetate |
| Hydrocortisone |
Neutral |
C21H30O5 |
362.5 |
1.61a |
12.59, -2.80* |
Ethyl acetate |
| Prednisolone |
Neutral |
C21H28O5 |
360.4 |
1.62a |
12.59, -2.90* |
Ethyl acetate |
| Pindolol |
Base |
C14H20N2O2 |
248.3 |
1.75a |
9.25 |
Ethyl acetate |
| Dexamethasone |
Neutral |
C22H29FO5 |
392.5 |
1.83a |
12.42, -3.30* |
Ethyl acetate |
| Corticosterone |
Neutral |
C21H30O4 |
346.5 |
2.02b |
13.86, -0.26* |
Ethyl acetate |
| 4-Propylbenzoic acid |
Acid |
C10H12O2 |
164.2 |
2.34b |
4.40 |
95:5 Dichloromethane/ IPA |
| Naproxen |
Acid |
C14H14O3 |
230.3 |
3.04b |
4.10 |
95:5 Dichloromethane/ IPA |
| 4-Pentylbenzoic acid |
Acid |
C12H16O2 |
192.3 |
3.12b |
4.40 |
95:5 Dichloromethane/ IPA |
| Ketoprofen |
Acid |
C16H14O3 |
254.3 |
3.12a |
4.45 |
95:5 Dichloromethane/ IPA |
| Propranolol |
Base |
C16H21NO2 |
259.3 |
3.48a |
9.42 |
3:1 Hexane:Ethyl acetate |
| Nortriptyline |
Base |
C19H21N |
263.4 |
3.90a |
9.70 |
3:1 Hexane:Ethyl acetate |
| Ibuprofen |
Acid |
C13H18O2 |
206.3 |
3.97a |
5.30 |
95:5 Dichloromethane/ IPA |
| Niflumic acid |
Acid |
C13H9F3N2O2 |
282.2 |
4.43a |
1.90, 5.50* |
95:5 Dichloromethane/ IPA |
| Diclofenac |
Acid |
C14H11Cl2NO2 |
296.1 |
4.51a |
4.15 |
95:5 Dichloromethane/ IPA |
| Protriptyline |
Base |
C19H21N |
263.4 |
4.70a |
10.50 |
3:1 Hexane:Ethyl acetate |
| Imipramine |
Base |
C19H24N2 |
280.4 |
4.80a |
9.40 |
3:1 Hexane:Ethyl acetate |
| Desipramine |
Base |
C18H22N2 |
266.4 |
4.90a |
10.40 |
3:1 Hexane:Ethyl acetate |
| Amitriptyline |
Base |
C20H23N |
277.4 |
4.92a |
9.40 |
3:1 Hexane:Ethyl acetate |
Sample preparation methods
Plasma preparation
Three distinct quantities of pig plasma (Sigma-Aldrich) were spiked to a concentration of 1 μg/mL with acidic, basic and neutral compounds (Table 1) and allowed 30 minutes to reach equilibrium prior to being used for LLE and SLE procedures.
SLE method
Using different pre-treatment solutions, the plasma was diluted after each plasma sample was loaded into the well. This caused the pH of the solution to change to allow for optimal extraction with organic solvent on elution whilst also diluting samples to enable enhanced flow onto the plate.
For the pre-treatment solutions, acidic compounds were diluted in 2% formic acid in water, basic compounds in 5% ammonia in water, and neutral compounds used deionized water.
Neutral compounds are not affected by pH, so water was used to dilute the sample for better flow onto the Microlute® SLE plate to ensure optimum recoveries. 200 μL of the diluted plasma (100 μL of pig plasma + 100 μL of pre-treatment solution) was loaded onto the Microlute® SLE 200 mg plate (Cat no: PSLE200P-001) using 3 PSI of positive pressure for 5 seconds (Figure 3). It was subsequently allowed to load fully for a period of 5 minutes, allowing the complete absorption of the sample. For the extraction of the analytes from the SLE product, 2 x 500 μL of solvent (Table 1 gives the solvent used for each type of analyte) was added to each well and allowed to elute under gravity into a 1 mL collection plate (Cat no: 219250). After both elutions had reached completion, 10 PSI of positive pressure was applied to the plate for 30 seconds to complete the elution. The eluent was dried via evaporation using N2 with the Porvair Sciences Ultravap® Levante (Cat no: 500226) at 35°C and reconstituted in 200 μL of starting mobile phase as seen in the solvent composition sections of Tables 1, 2 and 3.
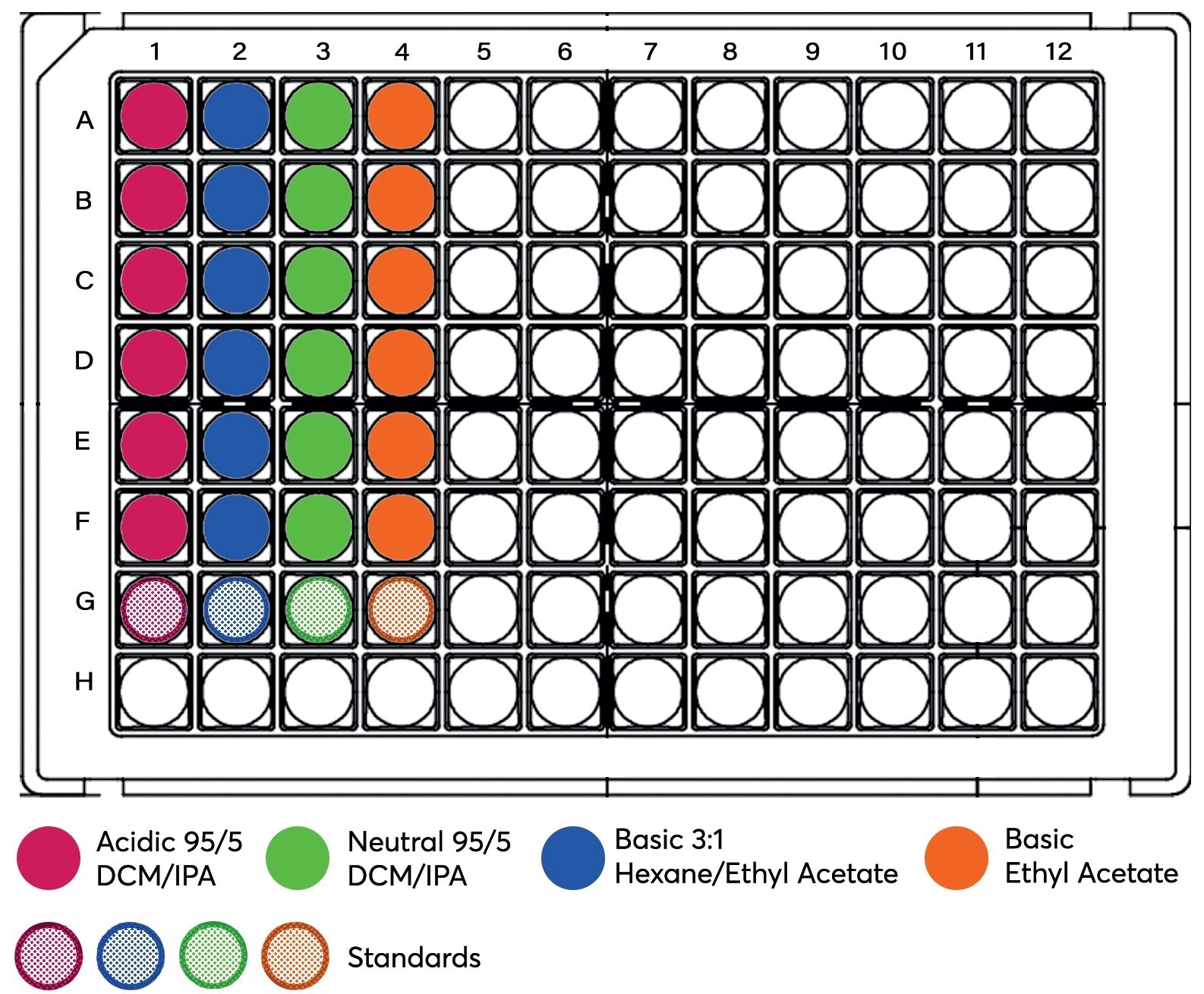
Figure 3. Schematic of all plasma samples, including matrix-matched standards (Row G) loaded onto the Microlute® SLE 200 mg. Image Credit: Porvair Sciences Limited
LLE method
LLE was carried out using 1.5 mL centrifuge tubes. To 200 μL of diluted plasma (as with SLE, the same pre-treatment was applied), 2 x 500 μL of solvent was added (the solvent used for each type of analyte is detailed in Table 1).
For each extraction, the samples were shaken by hand for 5 minutes and centrifuged at 10,000 RCF for 5 minutes. The organic layer was transferred carefully from the tube, using a glass pasteur pipette, into a collection plate (Cat no: 219250). It was then evaporated to dryness with N2 with the Porvair Sciences Ultravap® Levante (Cat no: 500226) at 35 °C and reconstituted in 200 μL of starting mobile phase.
Results and discussion
Chromatography
Chromatograms of basic, acidic and neutral analytes are represented in figures 4, 5 and 6 respectively. A Raptor Biphenyl (30 mm x 2.1 mm, 1.8 μm) column was used to separate the different classes of compounds with three different chromatographic methods (Table 1777), all in under 10 minutes.
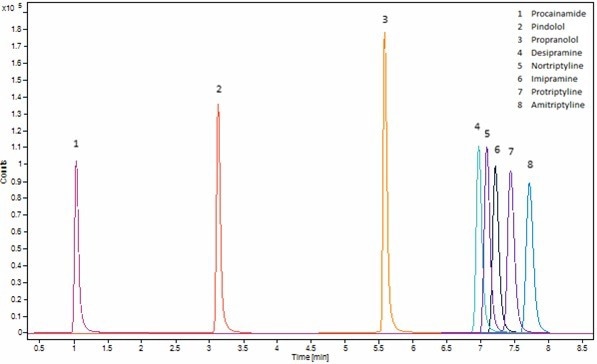
Figure 4. Chromatogram of matrix match basic analytes standard at 0.5 μg/mL. Method is shown in Table 1 (Basic) and SIM ion used is shown in Table 3. Image Credit: Porvair Sciences Limited
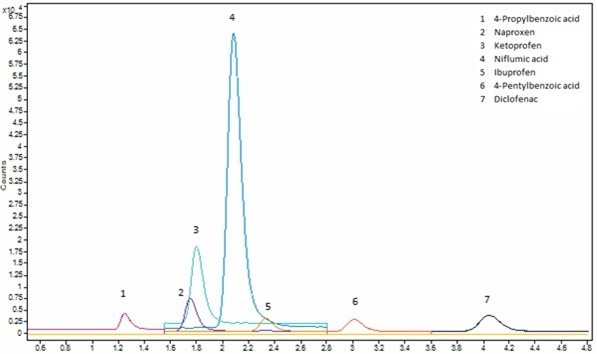
Figure 5. Chromatogram of matrix match acidic analytes standard at 0.5 μg/mL. Method is shown in Table 1 (Acidic) and SIM ion used is shown in Table 3. Image Credit: Porvair Sciences Limited
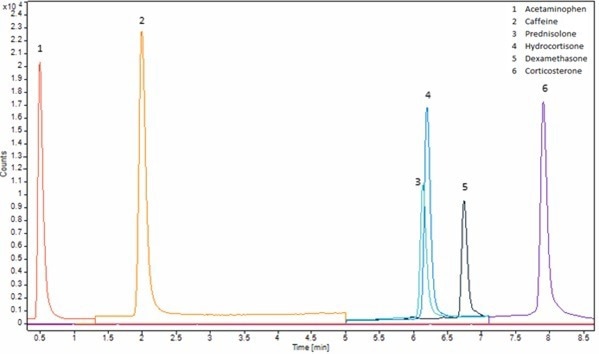
Figure 6. Chromatogram of matrix match neutral analytes standard at 0.5 μg/mL. Method is shown in Table 1 (Neutral) and SIM ion used is shown in Table 3. Image Credit: Porvair Sciences Limited
Recovery and reproducibility
Analyte recovery was calculated using the following equation:
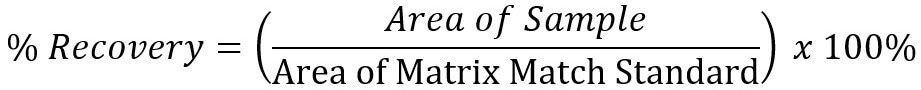
Analyte reproducibility was measured using percent relative standard deviation, which was calculated using the following equation:
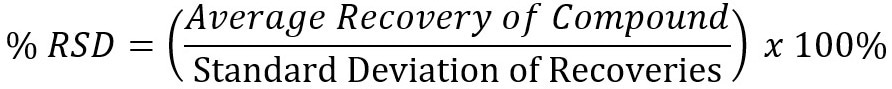
Figure 7 gives the mean recoveries for SLE and LLE side by side. Microlute® SLE shows improved reproducible recoveries across the analytes from each class (acidic, basic and neutral). Across all analytes tested, the average recovery was 94±6%. The recoveries were ranged between 89% and 106%.
The recoveries for LLE were not as high, with an average of 87±7%, with recoveries in the 79-98% range. Hence despite producing some high recoveries, results are on average lower than SLE and showed less reproducibility. LLE recoveries could be improved by employing a third extraction on the sample. However, this would further increase the sample processing time and would not provide a direct comparison to SLE. Issues may also arise from the introduction of matrix effects due to the further extraction of unwanted compounds, which can affect the ionization in the instrument’s source.
When comparing the two techniques, SLE recovery equals or betters that of LLE, and was the case for all analyte classes – acidic, basic and neutral. This is because of the better extraction efficiency present in the Microlute® SLE, providing a more effective solution to improve recoveries in a shorter period with less repetitive processes in place. Such results were made particularly clear when looking at the more hydrophilic compounds (caffeine and acetaminophen), which show a 10 - 20% increase in recovery in the SLE method.
Results for reproducibility are shown in Figure 8, which is represented as percent relative standard deviation (% RSD). A lower % RSD value indicates a more reproducible recovery. Reproducibility is a key metric in allowing confidence in results.
Having high recovery but with low reproducibility could cause the result to be question, and it may need to be acquired again. The average % RSD for the SLE recoveries was 3.2%, ranging between 0.7% and 6.9%. These values showed SLE to be more reproducible than the LLE work, which averaged 4.8% and with values in the range of 1.2% - 12.5%. This difference is most likely the result of LLE having more steps that rely on the technique of the analyst to ensure reproducible sample-to-sample extraction steps – shaking and then extracting the organic layer.
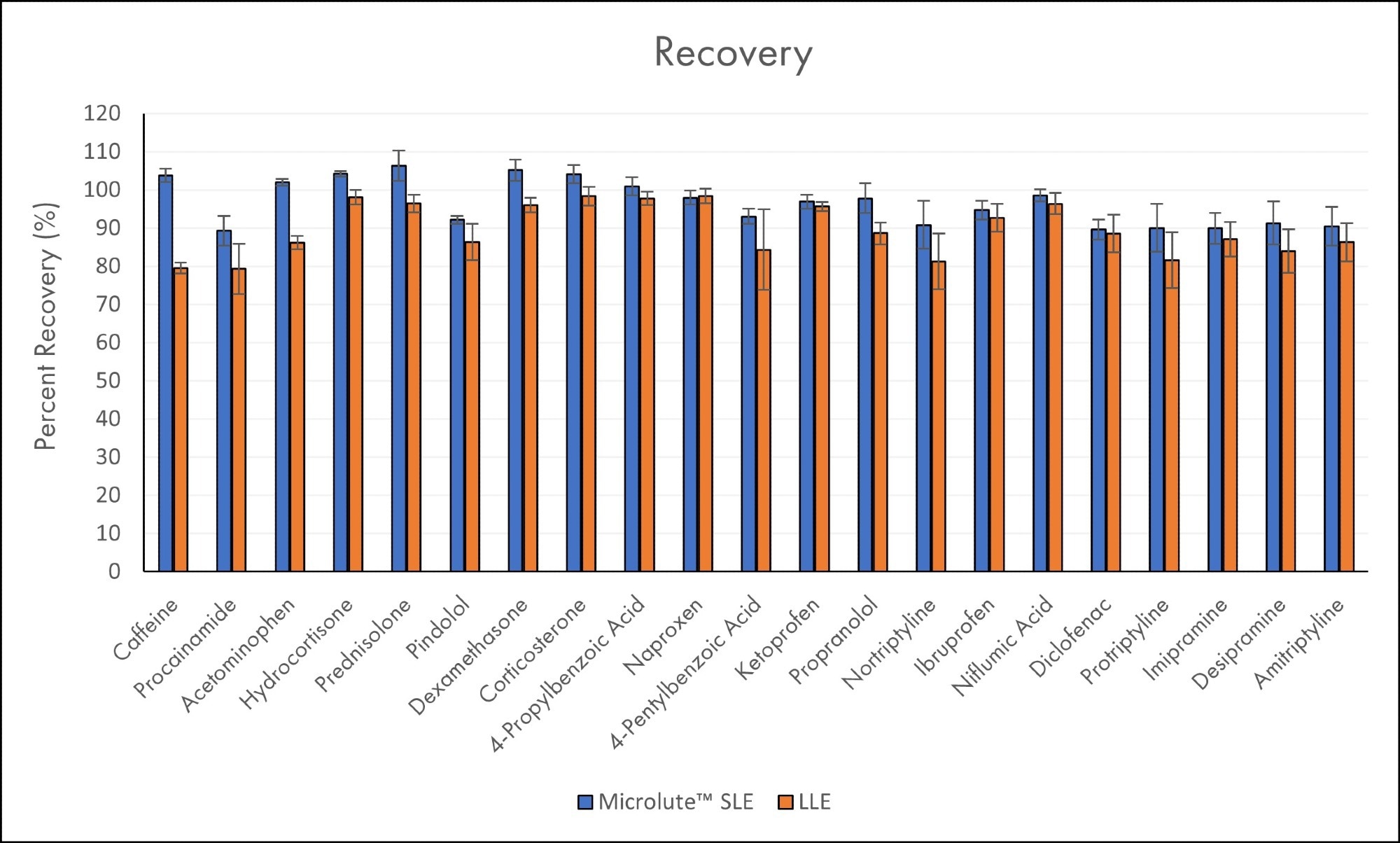
Figure 7. Mean recoveries for all analytes in plasma samples using Microlute® SLE 200 mg and LLE (N=6). The error bars represent the standard deviations of the recovery results. Analytes are ordered in increasing Log P values. Image Credit: Porvair Sciences Limited
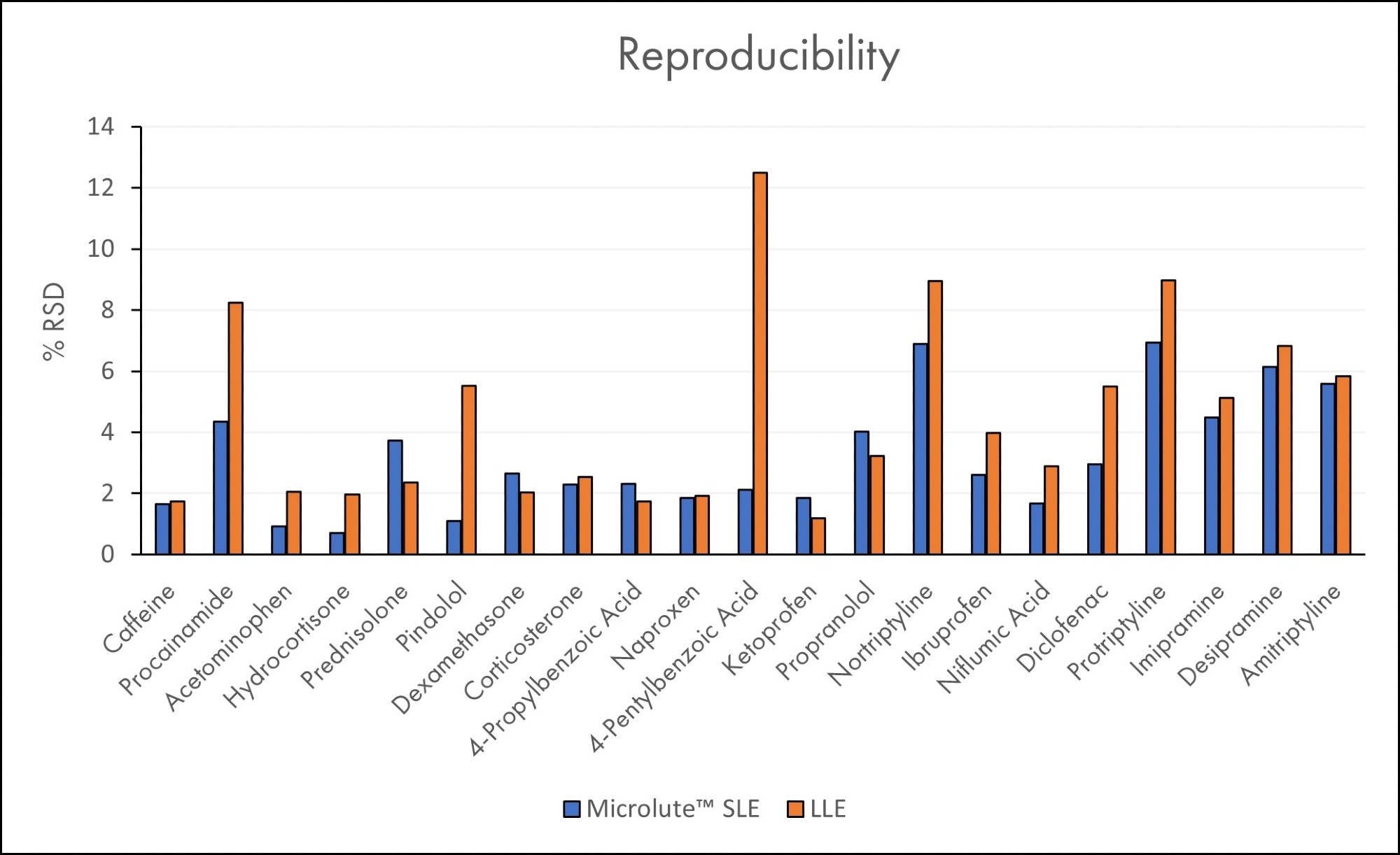
Figure 8. Relative Standard Deviations (%RSD) for all analytes in plasma samples using Microlute® SLE 200 mg and LLE (N=6). Analytes are ordered in increasing Log P values. Image Credit: Porvair Sciences Limited
Processing time
LLE is also outperformed by SLE with respect to the time taken to perform the sample preparation. Figure 9 illustrates the difference in processing times for each sample preparation method. Preparing 96 plasma samples for SLE is three times quicker than preparing an equivalent amount for LLE (40 minutes and 129 minutes, respectively). The lack of labor-intensive steps needed for SLE, such as sample shaking and transfer of organic solvent layers in LLE, is largely responsible for the Tim saved in SLE.
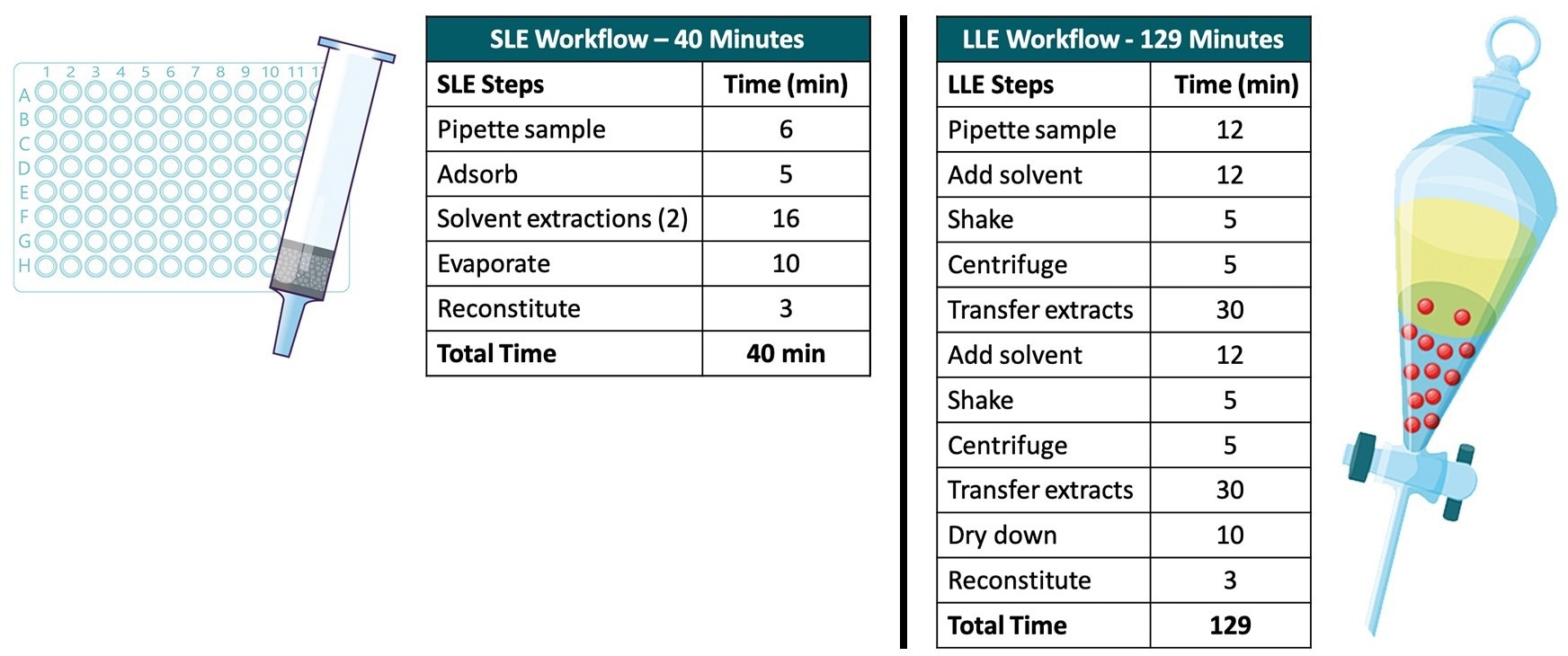
Figure 9. Schematic of all plasma samples including matrix-matched standards (Row G) loaded onto the Microlute® SLE 200 mg. Image Credit: Porvair Sciences Limited
Conclusion
This application note compared SLE methods with equivalent LLE methods for reproducibility, recovery and methodology using pig plasma on various compounds from different classes; acidic, basic and neutral. The comparison demonstrated that the Microlute® SLE plate is superior in both performances (obtaining higher and more reproducible results) and the time taken to perform the sample preparation, compared to the equivalent LLE method.
Using the Microlute® SLE plate allows increased sensitivity and confidence in the results. With the ability for high throughput by eliminating the need for the labor-intensive steps associated with LLE, it should be the ideal sample preparation method for any analyst.
References
- Ramirez C, Peters K. Extraction Techniques for Food Processing. ED-Tech Press; 2018.
About Porvair Sciences
Porvair Sciences, specialists in the manufacture of microplate products, serve Life Sciences, Biotechnology, R&D and Molecular Biology with microplate solutions for all applications, from sample preparation to high throughput screening via our global distributor network.
Our range includes vacuum manifolds, sealers, evaporators and microtiter plates in all popular styles; deep well and shallow well storage plates, assay plates, luciferase reporter gene plates and liquid handling reagent reservoirs. We also provide custom microplate products for life science research. Our vacuum manifolds, essential for 96-well SPE sample preparation, are designed to work with most popular filter plates, including Waters, Millipore, Qiagen, Whatman, GE Healthcare, Varian, Biotage and, of course, Porvair.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.