Solid-phase extraction (SPE) is the gold standard for sample preparation in chromatographic analysis. It is used to capture, clean, and concentrate samples while removing interfering compounds, helping users attain reliable and sensitive chromatographic data.
Conventionally, SPE products are loose packed with active resin between porous frits. However, these products suffer from inconsistent resin mass, channeling, and voiding which can reduce recovery and result in poor reproducibility.
The Microlute® CSi products utilize a novel composite technology with a mixture of porous plastic and chromatographic SPE resin, designed to eliminate the issues caused by the loose packing of SPE products.
The following experiment compares SPE results from a C18 10 mg loose packed plate with a C18 10 mg composite plate (Microlute® CSi), both created using the same batch of C18 resin.
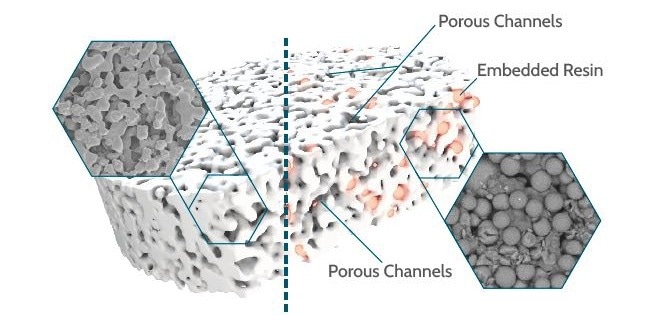
Figure 1. Schematic illustration of the composite technology. Image Credit: Porvair Sciences Limited
Methodology
A range of compounds consisting of acidic, basic, and neutral analytes were selected based on their intrinsic physical and chemical properties, such as pKa and LogP. Testing with a wide range of compounds enabled a more detailed comparison between each plate format.
Six replicates were tested from each compound class using a suitable SPE method.
The SPE followed a typical 10 mg reversed-phase method: condition: 500 µL MeOH; equilibration: 500 µL of H2O; load: 500 µL sample (pH adjusted to neutralize any charge on acidic and basic compounds); wash: 500 µL of H2O; elute: 2 x 250 µL MeOH (pH adjusted to charge the acidic and basic compounds).
The eluted samples were dried and reconstituted before being analyzed with an Agilent 1260 HPLC attached to an Agilent Single Quadrupole MSD.
Table 1. Properties of the analytes used in testing 1. Source: Porvair Sciences Limited
| Compound |
LogP |
pKa |
Analyte Type |
| Atenolol |
0.16 |
9.60 |
Basic |
| Pindolol |
1.75 |
9.25 |
Basic |
| Dexamethasone |
1.83 |
-3.30, 12.42 |
Neutral |
| Corticosterone |
2.09 |
-0.26, 13.86 |
Neutral |
| Carbamazepine |
2.77 |
-3.80, 15.96 |
Neutral |
| Ketoprofen |
3.12 |
4.45 |
Acidic |
| Naproxen |
3.18 |
4.15 |
Acidic |
| Niflumic acid |
4.43 |
5.30 |
Acidic |
| Propranolol |
3.48 |
9.42 |
Basic |
| Nortriptyline |
3.90 |
9.70 |
Basic |
| Desipramine |
4.90 |
10.40 |
Basic |
Results and discussion
Recovery
The results demonstrated that both plates performed well in analyte recovery across all 11 compounds. Average recoveries of 91% for the composite and 88% for the loose packed plates validate the utility of the SPE technique in sample preparation for chromatography.
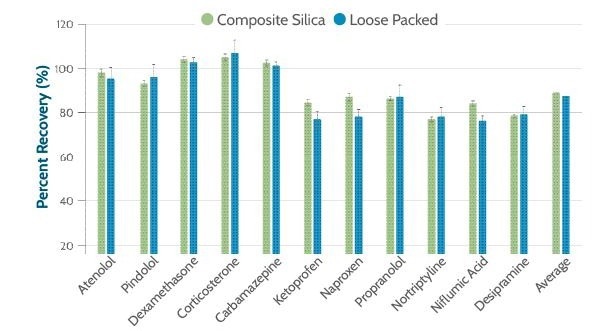
Figure 2. Percent recovery for each analyte using an average recovery calculated from six replicates. Image Credit: Porvair Sciences Limited
Reproducibility
The difference in reproducibility between the two plates was substantial; a significant improvement was evident when using the composite plate.
This could be demonstrated by calculating an average percentage for relative standard deviation (%RSD) across the compounds tested. The loose packed plate had an RSD of approximately 6%, while the composite plate had an RSD of less than 2% (Microlute® CSi).
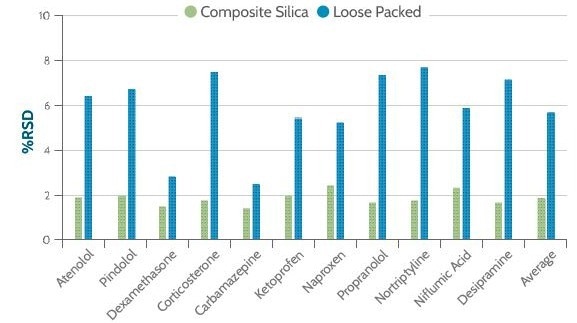
Figure 3. Percent relative standard deviation for recovery for each analyte using six replicates. Image Credit: Porvair Sciences Limited
Figure 3 shows the composite silica plate had markedly higher reproducibility for all 11 compounds tested. Poorer recovery and reproducibility in the loose packed plate can be attributed to inconsistencies in the packing of chromatographic media, which is inherent to this process (Figure 4).
The unique immobilization technology used in Microlute® CSi guarantees consistent bed weights and optimal liquid flow through every well, plate after plate. This leads to predictable interaction of solutions and analytes with the active chromatography resin.
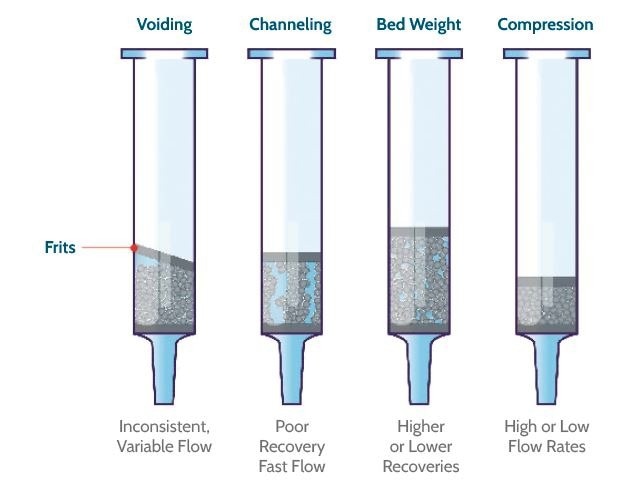
Figure 4. Common problems with traditional loose packed products. Image Credit: Porvair Sciences Limited
Conclusion
The 10 mg Microlute® CSi C18 composite silica plate for SPE offered excellent reproducibility and recovery for all the compounds assessed in this study. The uniformity of the composite technology enables an even distribution of active media and maximizes interactions throughout the structure, resulting in exceptionally reproducible data.
The novel composite format enhances recovery and can provide up to three times better reproducibility than the traditional loose packed plate formats.
Acknowledgments
Produced from materials originally authored by James Edwards from Porvair Sciences.
References and further reading
Drugbank, [Online]. Available at: https://go.drugbank.com/ (Accessed 2024)
About Porvair Sciences
Porvair Sciences are global leaders in the manufacturing and development of cutting-edge porous plastic materials and microplate technologies for the biotechnology and life science industries. From microplates and assay kits to automated laboratory equipment, our company is committed to the creation of workflow solutions with high quality products for improved analysis. Offering customers a diverse portfolio dedicated to high quality sample preparation, our innovative products are designed to increase productivity and accelerate scientific discovery with integrity.
Microlute®
The Microlute® range of sample preparation products were designed with reproducibility in mind. At the heart of the range are products with a unique hybrid structure, comprising a porous plastic disc with embedded active materials to enhance sample cleanup and analyte recovery. Discover the range of sample preparation solutions including solid phase extraction (SPE), phospholipid removal (PLR) and supported liquid extraction (SLE).
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.