Introduction
On behalf of Porvair Sciences R&D, the Department of Chemistry, University of Kent conducted a comprehensive study of commercial deep well microplates and this has been detailed in this article. In a previously independent report by Porvair Sciences, entitled “Extractables Testing in Deep Well Plates” [Castleman et al, Porvair Sciences 2005], the researchers concluded that the deep well microplates contained substantial levels of extractable compounds due to the use of lower grade polypropylene polymer.
In the present study, significant contamination levels were again found in many microplate samples from a range of manufacturers. This study widens the scope of prior studies and analyzes not only “natural” polymer microplates, but also black and colored plates from a much larger range of manufacturers than before.
The study was performed in early 2013 and offers data on a broad variety of microplates from numerous manufacturers in the USA, Europe and China. According to mass spectral data, persistent contamination from various compounds in the raw polymer master batch is still present in a number of the microplates tested.
Background
The impact of extractables identified in this report is complicated and is dependent on exactly which application the plate has been developed for. It is, however, obvious that extraneous fluorescence signals are caused by long chain hydrocarbon contaminants in any spectroscopic study and these contaminants may also result in false positives in large-scale screens examining stasis or apoptosis in whole cells.
In forensic applications, these unwanted molecules can mask drugs of abuse or their metabolites and lead to ion suppression, which results in inaccurate quantification. It is important for researchers who use deep-well microplates to conduct regular plate screening if they are to avoid this contamination. It is possible to carry out in-house testing or the deep-well microplates can be selected from highly regarded firms who are able to guarantee that contaminating compounds are not present in their products.
Method
The method followed is listed below:
- The researchers obtained deep-well microplate samples for testing from all key manufacturers.
- From each batch, a new unused plate was chosen and a stream of clean and dry compressed air was applied to eliminate any particulates that had accumulated.
- A suitable quantity of HPLC-grade methanol was incubated overnight in three wells in each sample plate in order to test for extractable contamination and polymer leachate.
- As an internal standard, methanol was spiked with 10µg/ml of caffeine.
- A friction seal was used to seal the plates, which were left to stand overnight.
- After being incubated overnight, a GC-MC system with spit-less injection at 250°C was used to analyze 1µl aliquots of each well sample.
- Using the appropriate temperature gradient, separation was carried out on a capillary column. Positive ion EI-MS was used for detection.
- To simplifying the complete data set, results from each of the three wells per plate tested were put together and averaged.
Results
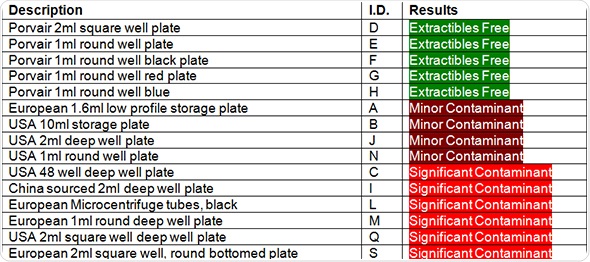
In the figures, plates that do not have any measurable contamination are shown in green. Minimal contamination, which is defined as just one contaminant and not necessarily present in all three tested wells, are shown in yellow. The plates that performed the worst and showed significant contamination are shown in red.
Table 1. Contamination in different manufacturer’s samples of deep well microplates
Contaminant Identification
The contaminants extracted were evaluated using an NIST database to identify the chemicals present. The chemicals were found to belong to a group that act as mould flow agents and plastic enhancers.
In certain products, other chemicals were seen at lower levels but they were not fully identified in this study. It is probable that the chemicals identified are included in the polymer mix early on in order to enhance ease of moulding and speed up cycle time of the mould, to generate the products required.
Recommendations
Recommendations made by the researchers include the following:
- Deep-well microplates that are used for long-term sample storage in organic solvents should be examined on a regular basis to check for extractable contamination.
- It is essential to understand this problem before stored samples or compounds are studied any further.
- During method validation, purity certificates should be obtained from the manufacturer and where this is not feasible, extractable testing should be performed to prevent the risk of contamination.
Conclusion
It can be concluded from the results that despite the availability of good quality medical-grade polypropylene for deep well microplate production, over 50% of the tested plates showed some form of contamination, usually caused by extractable compounds leaking from the plastic.
As with the 2005 independent lab tests, none of Porvair Sciences deep-well plates were found to be contaminated.
In this 2013 study, a considerable range of deep well plates from several suppliers were found to be contaminated with extractables. This leads us to the conclusion that the microplates were produced using a low-grade polypropylene. This kind of low-grade polypropylene often contains mould release agents or flow modifier additives that are used to make the manufacturing process easier and help free the moulded microplate from the mould.
A significant aspect of this study is the use of methanol. Being a polar solvent, methanol can easily dissolve tiny molecules and has been shown to extract most nonpolymer bound compounds from polypropylene microplates. It is also an ideal solvent for GC-MS. However, it is likely even more extractables would be observed if a more powerful solvent still such as DMSO was used.
Acknowledgement
Produced from articles authored by Stephen Knight and Tony Castleman, Porvair Sciences Ltd, Leatherhead, UK.
About Porvair Sciences
Porvair Sciences, specialists in the manufacture of microplate products, serve Life Sciences, Biotechnology, R&D and Molecular Biology with microplate solutions for all applications, from sample preparation to high throughput screening via our global distributor network.
Our range includes vacuum manifolds, sealers, evaporators and microtiter plates in all popular styles; deep well and shallow well storage plates, assay plates, luciferase reporter gene plates and liquid handling reagent reservoirs. We also provide custom microplate products for life science research. Our vacuum manifolds, essential for 96-well SPE sample preparation, are designed to work with most popular filter plates, including Waters, Millipore, Qiagen, Whatman, GE Healthcare, Varian, Biotage and, of course, Porvair.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.