Sponsored Content by SepmagReviewed by Maria OsipovaMay 9 2024
Once the required magnetic force is defined, scaling up production with a constant magnetic force separation device becomes a rather straightforward task. After validating the magnetic force at a small scale, you can apply the same force value to larger systems, even across different magnetic separation systems.

Image Credit: Sepmag
As the conditions are consistent, this approach guarantees efficiency without losses and ensures batch consistency without irreversible aggregation.

Figure 10.1. Sepmag Q1 L equipment and monitoring software. Image Credit: Sepmag
In constant magnetic force systems, the approved magnetic force value is converted to a constant separation speed. This is because the viscosity of the buffer generates a competition between the magnetic force and the drag force. When the magnetic force is radial, the separation time required is proportional to the vessel diameter (Figure 3)
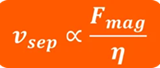
Figure 10.2. Where νsep is the separation time, Fmag is the magnetic force, and η is the viscosity. Image Credit: Sepmag
Development and validation can be monitored by measuring changes in absorbance (opacity) during separation.
This method offers users valuable insights into batch-to-batch consistency, the effectiveness of re-suspension protocols, and the impact of changes to the buffer. Additionally, it provides an objective means to determine the optimal separation time for a specific vessel and magnetic force value.

Figure 10.3. Equation for scaling up the volume while keeping the magnetic force constant, where Rvessel is the radius of the vessel. Image Credit: Sepmag
When scaling up, for example, from milliliters to 1 or 2 liters, maintaining a constant magnetic force is relatively straightforward. The working volume scales up as approximately (Rvessel)3.
This scaling is exact if the height-to-diameter ratio remains constant, but the separation time is proportional to the Rvessel. Magnetic separation productivity (volume of suspension processed per unit time) increases with (Rvessel)2.
Although there is no need to scale for production purposes, there are still many benefits of scaling up the batch volume, including simplified batch validation. A constant magnetic force also ensures within-batch consistency.
This implies that validating a single large batch is easier and safer than processing several batches and validating each one separately to assure batch consistency.
The benefits of working at a larger scale create a requirement for larger magnetic beat separation systems. Keeping the magnetic force constant for bigger vessels poses several technological challenges.
To maintain the same magnetic force at increasing volumes, the permanent magnet’s weight must also increase exponentially. For vessels larger than 5 L, utilizing the same magnetic force as used for smaller vessels becomes unfeasible in terms of weight and cost.
Users can also choose to slightly reduce the magnetic force for very large quantities. At realistic (i.e., practical) permanent magnet weights, the decreased magnetic force causes a slower separation speed.
The separation period is thus not only proportional to the vessel diameter but also inversely proportional to the magnetic force. In absolute terms, the separation time is rapid enough to support scaling up production.
Scaling up with traditional separators enhances the possibility of irreversible aggregation (the key constraint), particularly when coating magnetic beads for IVD because larger magnets require a greater retention force.
If a constant magnetic force strategy is used for scaling up, the reduction in magnetic force also reduces the likelihood of aggregation.

Figure 10.4. Estimate value of the separation time tsepB on a vessel of radius RvesselB in a Magnetic Bead Separation Systems with constant magnetic force FmagB, calculated from the separation value tsepA values of a vessel with radius RvesselA in a system with constant magnetic force FmagA. Image Credit: Sepmag
Estimating separation time in two different separators with different constant magnetic forces is quite straightforward.
In contrast to the separation time measured experimentally in the first device, the separation time in the second device is inversely proportional to the magnetic force values and directly proportional to the ratio of the vessel diameters (Figure 4).
If the magnetic force in the second system is constant (as in certain volume ranges), the separation time correlates to the vessel diameter. If the second (typically bigger) system has a smaller force than the first, users should account for the separation force.
Using constant magnetic force magnetic bead separation devices ensures within-batch consistency. This also allows for a smooth transition of procedures to other volumes, making it easy to scale up production as necessary.
About Sepmag 
Sepmag develops smart and scalable magnetic bead separation equipment for the international diagnostics market and for any user of magnetic bead separation techniques.
Sepmag's innovative Smart & Scalable Magnetic Bead Separators are designed to deliver unparalleled control and efficiency across all volumes, preventing bead aggregation, minimizing material loss, monitoring and keeping records for Quality Control purposes, and maximizing safety.
These benefits are applicable through a range of laboratory settings from R&D facilities to large scale production processes. Sepmag is based in Barcelona and sells in North America, Europe and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.