Sponsored Content by SepmagReviewed by Maria OsipovaMay 9 2024
Magnetic bead technology has provided many new possibilities in the field of life sciences, enabling applications such as nucleic acid purification and protein isolation.
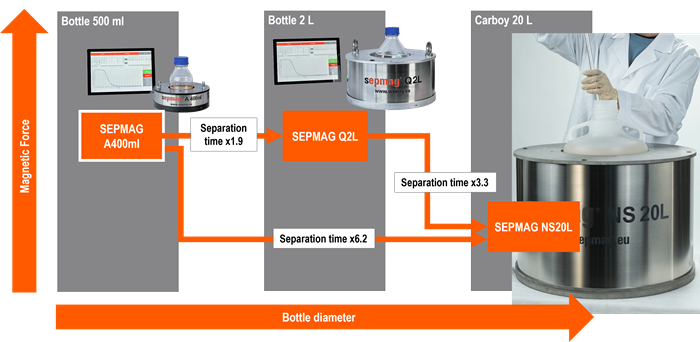
Image Credit: Sepmag
The absence of standardized technologies for separating proteins or molecules from complex biomaterials has slowed the transition from research and development (R&D) to large-scale production.
This limitation has posed several issues, especially in industries such as in vitro diagnostic (IVD) reagent manufacturing and magnetic bead production, where scaling up methods from R&D to production has been a difficult task.
This article looks at how standardizing magnetic bead separation settings at the R&D level may assist in building production-ready protocols. This standardization will revolutionize magnetic separation, ultimately simplifying and speeding up large-scale production.
Overcoming challenges in protocol transfer
The lack of standardization in magnetic bead separation settings has historically made it difficult to smoothly transfer techniques from R&D to production. In conventional magnetic bead separation systems that employ classical magnetic separators or basic magnets, magnetic force changes with distance.
This has presented considerable issues in magnetic bead separation techniques, resulting in uneven separation conditions across various volumes and vessel geometries. As a consequence, protocols designed for small-scale R&D applications are ineffective in large-scale manufacturing.
The role of constant and well-defined magnetic force
The key to simplifying protocol transmission lies in the utilization of Smart & Scalable magnetic bead separators, which provide a consistent and well-defined magnetic force.
Unlike typical separators, these novel devices apply a uniform magnetic force across the working volume, allowing constant separation conditions irrespective of vessel geometry or size.
By maintaining a constant magnetic force, the risk of bead aggregation is minimized, ensuring efficient separation without compromising the quality of the final product.
Standardized conditions for enhanced monitoring
Standardized magnetic bead separation settings not only improve protocol transmission but also allow real-time monitoring of the separation process in magnetic separators with measuring technologies like Sepmag.
Real-time monitoring is achieved by measuring the suspension’s absorbance. This capability enables researchers to accurately calculate separation time and evaluate the effects of different variables, such as buffer conditions and suspension composition.
This information can then be utilized to forecast separation times at larger volumes, ensuring scalability without requiring complex engineering projects.
Simplified scaling with real-time validation
With standardized magnetic bead separation conditions in place, process scale-up is fairly simple. Researchers can easily migrate from R&D to production by supplying specifications such as magnetic force values and vessel diameters to Smart & Scalable magnetic beat separator suppliers.
In addition, real-time absorbance monitoring enables validation of the scaled-up process, with the ability to fine-tune separation periods and other settings as needed.
Conclusion
The standardization of magnetic bead separation settings is a significant paradigm shift in life sciences, especially for production scalability.
Researchers can overcome the problems associated with protocol transfer from R&D to production by embracing Smart & Scalable magnetic beat separators and using constant and well-defined magnetic force principles.
This streamlines the scaling process while simultaneously ensuring consistency, effectiveness, and quality in the large-scale production of magnetic bead-based products.
With standardized conditions and real-time monitoring abilities, the opportunities for innovation and progress in the field of magnetic bead separation are endless, opening the path for breakthrough discoveries and applications in life science.
About Sepmag 
Sepmag develops smart and Scalable Magnetic Bead Separation equipment for the international diagnostics market and for any user of magnetic bead separation techniques.
Sepmag's innovative Smart & Scalable Magnetic Bead Separators are designed to deliver unparalleled control and efficiency across all volumes, preventing bead aggregation, minimizing material loss, monitoring and keeping records for Quality Control purposes, and maximizing safety.
These benefits are applicable through a range of laboratory settings from R&D facilities to large scale production processes. Sepmag is based in Barcelona and sells in North America, Europe and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.