Sponsored Content by SepmagReviewed by Maria OsipovaMay 9 2024
Magnetic bead technology for collecting biomolecules is becoming increasingly popular in the life sciences sector.

Image Credit: Sepmag
The use of magnetic beads in chemiluminescent immunoassays (CLIA) and molecular diagnostic tests has led to rapid enhancements in sensitivity and automation in these fields. This has increased the usefulness and viability of these tests in clinical and diagnostic settings.
During the COVID-19 pandemic, magnetic beads were frequently employed to facilitate immunoaffinity purification due to the large number of polymerase chain reaction (PCR) tests.
Magnetic beads have provided reliable assistance during the production of RNA-based vaccines, resulting in quicker and more efficient processes. Similarly, several biotech companies are looking into magnetic purification as a way of improving their downstream processes, particularly for proteins isolated from impure raw suspensions.
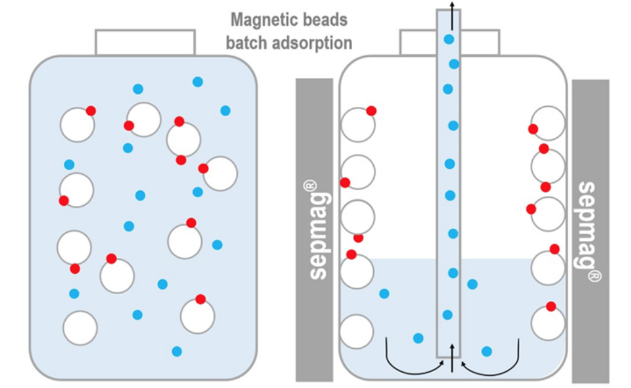
Much of this success can be attributed to the capability of magnetic beads to be mobile during biomolecule capture. However, they also become instantly immobilized when a magnetic force is applied.
This degree of control is not feasible with other commonly used methods and technologies, which makes dealing with biological suspensions with magnetic bead technology simpler and more accurate.
The advent of new applications for magnetic bead technology, however, has introduced many scientists to magnetic separation techniques for the first time, and there are several typical challenges that new users frequently encounter.
Additionally, after successfully employing magnetic bead-based biomolecule isolation at the research and development (R&D) scale, many new users face challenges when attempting to scale up to larger manufacturing processes. The distinct characteristics of magnetic bead technology compared to other technologies make these scale-up efforts particularly difficult for newcomers.
In the fields of reagents manufacturing and product purification, technical staff are tasked with standardization, safety, quality control, and scaling up protocols. Scientists must possess advanced skills in developing robust protocols that consider various factors, such as the surface chemistry of the beads, batch volumes and concentrations, buffer composition, and the incubation time and temperature.
However, scientists who are not familiar with essential magnetic properties might overlook critical parameters needed for magnetic separation protocols, jeopardizing the process's success. This oversight can severely complicate reproducing or scaling up from initial small-scale protocols, often creating bottlenecks in R&D departments during the transition to production.
Magnetic beads separation process. The magnetic beads coated with a ligand capture the target molecule. Once the suspension is introduced in a magnetic separator, the magnetic beads (with the captured target molecule) are immobilized in seconds and the supernatant can be completely removed. Image Credit: Sepmag
For over fifteen years, well-defined magnetic separation protocols have been used for in vitro diagnostic (IVD) reagent manufacturing and magnetic bead production. This enables manufacturers to effectively scale up from milliliter to tens of liter sized batches.
By incorporating some important magnetism concepts, it is possible to successfully work at scale with magnetic bead technology.
With this knowledge, scientist personnel can comprehensively characterize and validate magnetic separation procedures, therefore standardizing magnetic separation conditions and developing reliable protocols that can be readily transferred to larger volumes.
About Sepmag 
Sepmag develops smart and scalable magnetic bead separation equipment for the international diagnostics market and for any user of magnetic bead separation techniques.
Sepmag's innovative Smart & Scalable Magnetic Bead Separators are designed to deliver unparalleled control and efficiency across all volumes, preventing bead aggregation, minimizing material loss, monitoring and keeping records for Quality Control purposes, and maximizing safety.
These benefits are applicable through a range of laboratory settings from R&D facilities to large scale production processes. Sepmag is based in Barcelona and sells in North America, Europe and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.